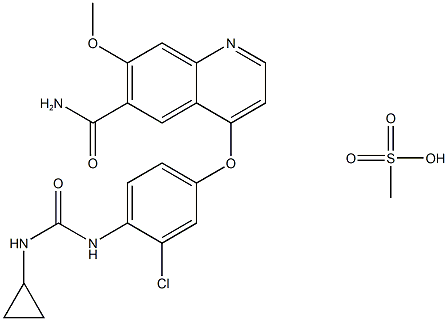
lenvatinib Mesylate
- CAS No.
- 857890-39-2
- Chemical Name:
- lenvatinib Mesylate
- Synonyms
- Lenvatinib mesilate;4-[3-Chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide monomethanesulfonate;CAT#A863437;E7080 Mesylate;envatinib MesyL;Lenvatinib Meylate;Levatinib mesylate;Lenvatinib Mesylat;levitinib mesylate;E7080;E-7080;E 7080
- CBNumber:
- CB42651170
- Molecular Formula:
- C22H23ClN4O7S
- Molecular Weight:
- 522.95862
- MOL File:
- 857890-39-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/26 17:49:17
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
lenvatinib Mesylate Chemical Properties,Uses,Production
Description
Lenvatinib mesylate (lenvatinib) is an oral targeted therapy medicine known as a receptor‐type tyrosine kinase inhibitor. It is a small-molecule drug (a drug that can enter cells easily), which was developed at Eisai in 2015. It was approved by the FDA in 2015 for the treatment of differentiated thyroid cancer that is either locally recurrent, metastatic, or progressive and did not respond to radioactive iodine treatment. In May 2016, the FDA approved the drug as a combination therapy with everolimus for the treatment of advanced renal cell carcinoma. Because VEGF (and fibroblast growth factor receptors, known as FGFRs) are thought to play a role in cardiovascular signaling pathways, VEGF2R and FGFR inhibition are thought to be the mechanisms behind the primary side effect of lenvatinib mesylate, which is hypertension.
Uses
Lenvatinib mesylate is used in cancer:(1) Endometrial carcinoma that is advanced and got worse after other systemic therapies. It is used with pembrolizumab in patients whose cancer is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) and cannot be treated with surgery or radiation therapy.(2) Hepatocellular carcinoma (a type of liver cancer). It is used as the first treatment in patients whose cancer cannot be removed by surgery.(3) Renal cell carcinoma (a type of kidney cancer) that is advanced(4)Thyroid cancer in certain patients whose cancer got worse, came back, or has spread to other parts of the body and did not respond to treatment with radioactive iodine.(5) Lenvatinib Mesylate is used in preparation of anti-human CTLA4xPD-1 bispecific antibodies for diagnosis, prevention and treatment of tumor or anemia.
Lenvatinib mesylate is also being studied in the treatment of other types of cancer.
Definition
ChEBI: Lenvatinib mesylate is a methanesulfonate salt obtained by reaction of lenvatinib with one molar equivalent of methanesulfonic acid. A multi-kinase inhibitor and orphan drug used (as its mesylate salt) for the treatment of various types of thyroid cancer that do not respond to radioiodine. It has a role as an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor, a fibroblast growth factor receptor antagonist, an orphan drug, a vascular endothelial growth factor receptor antagonist and an antineoplastic agent. It contains a lenvatinib(1+).
Mechanism of action
Lenvatinib mesylate works by blocking proteins that signal cancer cells to multiply. It also blocks proteins that signal the formation of new blood vessels that are needed to support tumor growth. Blocking these signals keeps cancer cells from growing.
Pharmacokinetics
Lenvatinib is rapidly absorbed after oral administration, with tmax typically observed at 1 to 4 hours postdose. Food does not affect the extent of absorption but slows the rate of absorption. When administered with food to healthy subjects, peak plasma concentrations are delayed by 2 hours.
In vitro binding of lenvatinib to human plasma proteins was high and ranged from 98% to 99% (0.3 – 30 μg/mL, lenvatinib Mesylate). This binding was mainly to albumin with minor binding to α1-acid glycoprotein and γ-globulin. In vitro, the lenvatinib blood-to-plasma concentration ratio ranged from 0.589 to 0.608 (0.1 - 10 μg/mL, lenvatinib Mesylate). Lenvatinib is a substrate for P-gp and BCRP. Lenvatinib is not a substrate for OAT1, OAT3, OATP1B1, OATP1B3, OCT1, OCT2, or the BSEP.
Side effects
The most prevalent AEs were hypertension (77.8%), fatigue (55.6%), weight loss (51.9%).In addition, there may be fatigue or tiredness, rashes, redness, itching or peeling of the palms and soles of the feet, diarrhoea, nausea, constipation and heartburn.
Solubility in water
lenvatinib Mesylate is a white powder sparingly soluble in acetic acid and slightly soluble in water. It is very slightly soluble in 1,3-dimethyl-2-imidazolidinone and practically insoluble in acetonitrile, dehydrated ethanol, 1-propanol, 2-propanol, 1-octanol, and isopropyl acetate. In aqueous solutions, lenvatinib mesylate is slightly soluble in 0.1 mol/L HCl and practically insoluble in Britton-Robinson buffer, pH 3-11.
lenvatinib Mesylate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Beta Drugs | +91-9316590666 +91-7015991921 | Haryana, India | 34 | 58 | Inquiry |
| SHILPA MEDICARE LTD | 08532 - 238704 | New Delhi, India | 52 | 58 | Inquiry |
| SUN PHARMACEUTICAL INDUSTRIES LTD | (+91 22) 4324 4324 | New Delhi, India | 175 | 58 | Inquiry |
| DR REDDYS LABORATORIES LTD | +91-40-49002900 | New Delhi, India | 201 | 58 | Inquiry |
| MSN LABORATORIES PRIVATE LTD | +91 40 30438600 | New Delhi, India | 230 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
Related articles
- Synthesis and Application of Lenvatinib Mesylate
- Lenvatinib Mesylate is a multitarget tyrosine kinase inhibitor that may act on a variety of cancers.
- Aug 5,2022
857890-39-2(lenvatinib Mesylate)Related Search:
1of4
chevron_right




