Ammonium sulfamate
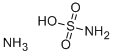
- CAS No.
- 7773-06-0
- Chemical Name:
- Ammonium sulfamate
- Synonyms
- AMS;AMMONIUM AMIDOSULFATE;Sulfamate;SULFAMIC ACID AMMONIUM SALT;Ammat;Amcide;Ammate;Ikurin;famate;Amicide
- CBNumber:
- CB4344641
- Molecular Formula:
- H6N2O3S
- Molecular Weight:
- 114.12
- MOL File:
- 7773-06-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 131-135 °C (lit.) |
|---|---|
| Boiling point | 160°C |
| Density | 1.769 |
| vapor pressure | 0Pa at 20℃ |
| Flash point | 160°C |
| storage temp. | no restrictions. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Crystalline Powder |
| color | White |
| PH | 5.0-6.0 (50g/l, H2O, 20℃) |
| Odor | Slight amine odor |
| PH Range | 4.5 - 6.0 |
| Water Solubility | 1950 g/L (20 ºC) |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,554 |
| Exposure limits |
ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 1500 mg/m3; TWA 10 mg/m3; TWA 5 mg/m3 |
| Stability | Stable. Incompatible with strong acids, strong oxidizing agents. |
| InChIKey | GEHMBYLTCISYNY-UHFFFAOYSA-N |
| LogP | -4.34 at 20℃ |
| CAS DataBase Reference | 7773-06-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Ammonium sulfamate(7773-06-0) |
| EPA Substance Registry System | Ammonium sulfamate (7773-06-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS09,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H335-H336-H302-H400 | |||||||||
| Precautionary statements | P301+P312a-P330-P501a-P273-P261-P264-P270-P271-P280-P301+P312+P330-P304+P340+P312-P305+P351+P338+P337+P313-P403+P233-P405-P501 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 22-50 | |||||||||
| Safety Statements | 24/25-22-61 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3 (total) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | WO6125000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29222100 | |||||||||
| Toxicity | LD50 orally in rats: 3.0 g/kg (Ambrose) | |||||||||
| IDLA | 1,500 mg/m3 | |||||||||
| NFPA 704 |
|
Ammonium sulfamate price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | A2585 | Ammonium sulfamate BioXtra, ≥98.0% | 7773-06-0 | 100G | ₹4979.5 | 2022-06-14 | Buy |
| Sigma-Aldrich | 51512 | Ammonium sulfamate PESTANAL?, analytical standard | 7773-06-0 | 100MG | ₹5033.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 51512 | Ammonium sulfamate PESTANAL?, analytical standard | 7773-06-0 | 100MG | ₹5033.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 228745 | Ammonium sulfamate ACS reagent, ≥98.0% | 7773-06-0 | 100G | ₹4070.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 228745 | Ammonium sulfamate ACS reagent, ≥98.0% | 7773-06-0 | 500G | ₹6419.23 | 2022-06-14 | Buy |
Ammonium sulfamate Chemical Properties,Uses,Production
Description
Ammonium sulfamate (or ammonium sulphamate) is a white crystalline solid, readily soluble in water. It is a salt formed from ammonia and sulfamic acid. Based on its good performance, it is widely used in industrial production and scientific research. Ammonium sulfamate is used as a herbicide, a composting accelerator in horticultural settings, a flame retardant, and so on.
Chemical Properties
Ammonium sulfamate is a white to yellow crystalline solid.
Uses
Ammonium sulfamate (AMS) is an inorganic herbicide used for control of woody plants and herbaceous perennials.
Application
Ammonium sulfamate could be widely used in pesticides, printing and dyeing, tobacco, building materials, textiles, and other industries. It could used as an analytical reagent. It could also used in the manufacture of fire-retardant compositions, for flameproofing textiles and paper products, and in the manufacture of weed-killing compositions. It is also used in electroplating solutions and could used for the generation of nitrous oxide gas.
Preparation
Ammonium sulfamate is prepared continuously, starting from ammonia and sulfur trioxide, in which liquid sulfur trioxide and liquid ammonia are introduced in a molar ratio NH3 /SO3 greater than 2.0:1 into a pressure reactor containing the reaction product in the molten state, excess ammonia being removed continuously from the reactor and the liquid sulfur trioxide being introduced into the melt.
General Description
Ammonium sulfamate is a white crystalline solid. Ammonium sulfamate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium sulfamate is used to flameproof fabrics and papers, in weed or brush killing products, and for other uses.
Air & Water Reactions
Water soluble. Hot water [Note: Elevated temperatures cause a highly exothermic reaction with water (in the presence of acid)] .
Reactivity Profile
Ammonium sulfamate is incompatible with the following: Acids, hot water [Note: Elevated temperatures cause a highly exothermic reaction with water (in the presence of acid).] .
Hazard
Hot acid solutions when enclosed may explode.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion causes gastrointestinal disturbances. Dust irritates eyes.
Fire Hazard
Special Hazards of Combustion Products: Toxic oxides of nitrogen may form in fires.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Somewhat explosive when heated or by spontaneous chemical reaction in a hot acid solution. A powerful oxidizer. When heated to decomposition it emits very toxic fumes of NH3, NOx, and SOx. See also SULFONATES and SULFAMIC ACID.
Potential Exposure
Ammonium sulfamate is used as a herbicide and in compositions for retarding flame in textiles and paper products; a softener for paper, cotton textiles.
Carcinogenicity
The oral LD50 values were 3900 mg/kg for rats and 5760 mg/kg for mice.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Crystallise it from water at room temperature (1mL/g) by adding EtOH and cooling. [Sisler & Audrieth Inorg Synth II 180 1946.]
Incompatibilities
Strong oxidizers, potassium, potassium chlorate, sodium nitrite, metal chlorates, and hot acid solutions. Elevated temperatures cause a highly exothermic reaction with water.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Dilute with water, make neutral with acid or base and flush into sewer with more water.
Ammonium sulfamate Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Nithyasri Chemicals (APURVA CHEMICALS) | +91-2512692130 +91-9323652199 | Maharashtra, India | 75 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3077 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| A. B. Enterprises | +91-8048077585 | Mumbai, India | 1396 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Nithyasri Chemicals (APURVA CHEMICALS) | 58 |
| JSK Chemicals | 58 |
| A.J Chemicals | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Central Drug House(P) Ltd. | 58 |
| LOBA CHEMIE PVT.LTD. | 58 |
| Triveni chemicals | 58 |
| A. B. Enterprises | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
7773-06-0(Ammonium sulfamate)Related Search:
1of4
chevron_right




