COBALT (II) SULFAMATE
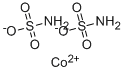
- CAS No.
- 14017-41-5
- Chemical Name:
- COBALT (II) SULFAMATE
- Synonyms
- t suL;famate hydrate;COBALT SULFAMATE;Cobaltoussulamate;cobalt sulphamate;COBALT SULFAMINATE;Cobalt disulfamate;Cobalt(II)sulfamat;cobalt disulphamate;COBALTOUS SULFAMATE
- CBNumber:
- CB5250068
- Molecular Formula:
- CoH4N2O6S2
- Molecular Weight:
- 251.1
- MOL File:
- 14017-41-5.mol
- Modify Date:
- 2023/6/20 22:47:09
| Density | 2.202[at 20℃] |
|---|---|
| form | Powder |
| Water Solubility | Soluble in water. |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| CAS DataBase Reference | 14017-41-5(CAS DataBase Reference) |
| EPA Substance Registry System | Cobaltous sulfamate (14017-41-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H317-H341 | |||||||||
| Precautionary statements | P201-P261-P280-P308+P313-P405-P501a | |||||||||
| TSCA | Yes | |||||||||
| NFPA 704 |
|
COBALT (II) SULFAMATE Chemical Properties,Uses,Production
Uses
Cobalt(II) sulfamate hydrate is used in photonic device fabrication.
General Description
COBALT (II) SULFAMATE is a reddish colored solid. COBALT (II) SULFAMATE is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit the spread to the environment. COBALT (II) SULFAMATE is used as a pigment and electroplating metals.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as COBALT (II) SULFAMATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Contact with wood or paper may cause fire [USCG, 1999]. Special Hazards of Combustion Products: Toxic oxides of nitrogen may form in fire.
Health Hazard
Inhalation causes shortness of breath and coughing; permanent disability may occur. Ingestion causes pain and vomiting. Contact with eyes or skin causes irritation.
COBALT (II) SULFAMATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shree Astavinayak Technology | 08048372896Ext 654 | Ahmedabad, India | 1 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | China | 12456 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | China | 8822 | 58 | Inquiry |
| Shanghai Longyu Biotechnology Co., Ltd. | +8613917842738 | China | 2534 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | China | 23556 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 24722 | 58 | Inquiry |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | China | 9322 | 58 | Inquiry |





