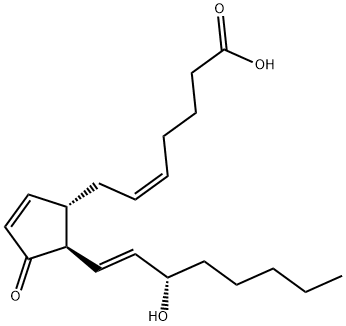
PROSTAGLANDIN J2
- CAS No.
- 60203-57-8
- Chemical Name:
- PROSTAGLANDIN J2
- Synonyms
- PGJ2;Aids058772;Aids-058772;PROSTAGLANDIN J2;PGJ2 (Prostaglandin J2);Prostaglandin J2 (PGJ2);9-deoxy-delta-9-prostaglandind2;Prostaglandin J2 Lipid Maps MS Standard;PROSTAGLANDIN J2, 5MG/ML IN METHYL ACETA;11-OXO-15S-HYDROXY-PROSTA-5Z,9,13E-TRIEN-1-OIC ACID
- CBNumber:
- CB4477024
- Molecular Formula:
- C20H30O4
- Molecular Weight:
- 334.45
- MOL File:
- 60203-57-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:09
| Boiling point | 521.7±50.0 °C(Predicted) |
|---|---|
| Density | 1.103±0.06 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | DMF: >100 mg/ml (from PGD2); DMSO: >50 mg/ml (from PGD2); Ethanol: >75 mg/ml (from PGD2); PBS pH 7.2: >2.7 mg/ml (from 15-deoxy-.DEL |
| pka | 4.75±0.10(Predicted) |
| form | Liquid |
| color | Colorless to light yellow |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319-H336 | |||||||||
| Precautionary statements | P210-P240-P241-P242-P243-P261-P264-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P312-P337+P313-P370+P378-P403+P233-P403+P235-P405-P501 | |||||||||
| Hazard Codes | Xn,Xi,F | |||||||||
| Risk Statements | 20/21/22-36/37/38-67-66-36-11 | |||||||||
| Safety Statements | 26-36-33-29-16 | |||||||||
| NFPA 704 |
|
PROSTAGLANDIN J2 Chemical Properties,Uses,Production
Description
Prostaglandin J2 (PGJ2) is formed from PGD2 by the elimination of the C-
Uses
PGJ2 (Prostaglandin J2) is a Prostaglandin D2 metabolite that has shown potent anti-neoplastic and antiviral activity.
Definition
ChEBI: A member of the class of prostaglandins J that consists of prosta-5,9,13-trien-1-oic acid substituted by an oxo group at position 11 and a hydroxy group at position 15 (the 5Z,13E,15S stereoisomer).
PROSTAGLANDIN J2 Preparation Products And Raw materials
Raw materials
Preparation Products
PROSTAGLANDIN J2 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Shanghai Aladdin Bio-Chem Technology Co.,LTD | 400-400-6206333 18521732826 | China | 25009 | 65 | Inquiry |
| TargetMol Chemicals Inc. | 15002134094 | China | 28070 | 58 | Inquiry |
| Shanghai Universal Biotech Co.,Ltd | 18768175414 | China | 24998 | 58 | Inquiry |
| Hubei Aiputi Bioengineering Co., Ltd. | 17762444226 | China | 2402 | 58 | Inquiry |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 18621169085 | China | 27996 | 58 | Inquiry |
| Cayman Chemical Company | (800) 364-9897 | United States | 6618 | 81 | Inquiry |
| Shanghai Yifei Biotechnology Co. , Ltd. | 021-65675885 18964387627 | China | 8736 | 58 | Inquiry |
60203-57-8(PROSTAGLANDIN J2)Related Search:
1of4
chevron_right




