Diazomethane
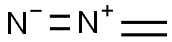
- CAS No.
- 334-88-3
- Chemical Name:
- Diazomethane
- Synonyms
- DIAZIRINE;azimethane;azimethylene;Diazomethane;DIAZOMETHANE IN ETHER;Methane, diazo-(8CI,9CI);Diazomethane ISO 9001:2015 REACH
- CBNumber:
- CB4852342
- Molecular Formula:
- CH2N2
- Molecular Weight:
- 42.03998
- MOL File:
- 334-88-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:26
| Melting point | -145° |
|---|---|
| Boiling point | bp -23° |
| Density | 1.45 g/cm3 |
| refractive index | 1.4180 (estimate) |
| form | Yellow gas |
| Odor | Musty odor (no accepted threshold value) |
| Exposure limits | TLV-TWA 0.2 ppm (0.38 mg/m3 ) (ACGIH) PEL-TWA 0.2 ppm (0.38 mg/m3 ) (OSHA). |
| IARC | 3 (Vol. 7, Sup 7) 1987 |
| EPA Substance Registry System | Diazomethane (334-88-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H350 |
| Hazard Codes | T |
| Risk Statements | 45 |
| Safety Statements | 53-45 |
| OEB | C |
| OEL | TWA: 0.2 ppm (0.4 mg/m3) |
| RIDADR | 1953 |
| Autoignition Temperature | 150 °C; impure material explodes at lower temperature |
| HazardClass | 2.3 |
| Toxicity | LCLO inhal (cat) 175 ppm (10 min) PEL (OSHA) 0.2 ppm (0.4 mg/m3) TLV-TWA (ACGIH) 0.2 ppm (0.4 mg/m3) |
| IDLA | 2 ppm |
Diazomethane Chemical Properties,Uses,Production
Chemical Properties
Diazomethane is a flammable, yellow gas or a liquid under pressure. Musty odor.
Uses
Powerful methylating agent for acidic Compounds such as carboxylic acids, phenols, enols. For syntheses with diazomethane see the reviews by Smith, Chem. Rev. 23, 193 (1938); Eistert, Z. Angew. Chem. 54, 99, 124 (1941) translated by Spangler in Newer Methods of Preparative Organic Chemistry (New York, 1948) p 513; J. S. Pizey, Synthetic Reagents vol. 2 (John Wiley, New York, 1974) pp 65-142.
Definition
ChEBI: The simplest diazo compound, in which a diazo group is attached to a methylene group.
General Description
Yellow gas with a musty odor. Highly toxic by inhalation Shipped as a liquid under pressure.
Air & Water Reactions
Reacts with water, releasing nitrogen, more stable in ether or dioxane.
Reactivity Profile
Diazomethane undergoes violent thermal decomposition. Above 200°C. the vapors may explode violently if rough glass surfaces are present. Explosions at low temperatures can occur if traces of organic matter are present. [J. Phys. Chem. 35:1403(1931)]. Produces explosions with alkali metals. Reacts with copper powder and to some extent all solid surfaces to produce nitrogen and solid white polymethylene. Reacts with dimethylaminodimethylarsine and trimethyltin in ether with vigorous foaming.
Health Hazard
Diazomethane vapor causes severe irritation of the skin, eyes, mucous membranes, and lungs. It is considered to be a substance with poor warning properties, and the effects of exposure may be delayed in onset. Symptoms of exposure may include headache, chest pain, cough, fever, severe asthmatic attacks, and pulmonary edema, which can be fatal. Exposure of the skin and mucous membranes to diazomethane may cause serious burns. Diazomethane is a powerful allergen. Prolonged or repeated exposure to diazomethane can lead to sensitization of the skin and lungs, in which case asthma- like symptoms or fever may occur as the result of exposure to concentrations of diazomethane that previously caused no symptoms. Chronic exposure to diazomethane has been reported to cause cancer in experimental animals, but this substance has not been identified as a human carcinogen. Note that diazomethane is often prepared in situ from precursors that may themselves be highly toxic and/or carcinogenic.
Fire Hazard
Pure diazomethane gas and liquid are readily flammable and can explode easily. A variety of conditions have been reported to cause explosions of diazomethane, including contact with rough surfaces such as ground-glass joints, etched or scratched flasks, and glass tubing that has not been carefully fire-polished. Direct sunlight and strong artificial light may also cause explosions of this substance. Violent reactions may occur on exposure of diazomethane to alkali metals.
Flammability and Explosibility
Pure diazomethane gas and liquid are readily flammable and can explode easily. A variety of conditions have been reported to cause explosions of diazomethane, including contact with rough surfaces such as ground-glass joints, etched or scratched flasks, and glass tubing that has not been carefully fire-polished. Direct sunlight and strong artificial light may also cause explosions of this substance. Violent reactions may occur on exposure of diazomethane to alkali metals.
Safety Profile
Confirmed carcinogen with experimental tumorigenic data. A poisonous irritant by inhalation. A powerful allergen. It can cause pulmonary edema and frequently causes hypersensitivity leading to asthmatic symptoms. Mutation data reported. Highly explosive when shocked, exposed to heat, or by chemical reaction. Undiluted liquid or gas may explode on contact with alkali metals, rough surfaces, heat (lOO°C), hgh-intensity light, or shock. When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of NOx. Incompatible with alkali metals; calcium sulfate.
Potential Exposure
Diazomethane is a powerful methylat- ing agent for acidic compounds, such as carboxylic acids, phenols and enols. It is used in pesticide manufacture and pharmaceutical manufacture.
Carcinogenicity
Diazomethane was administered to rats and mice by inhalation, dermal, or subcutaneous injection routes using concentrations of 0.1 or 3.3 mg/mL. Mice developed lung tumors following either dermal application or inhalation at both concentrations.
storage
diazomethane should preferably be handled in solution using glassware specially designated for diazomethane (e.g., with Clear-Seal joints) and should be used as soon as possible after preparation. Storage of diazomethane solutions (even at low temperature) is not advisable. All work with diazomethane should be conducted in a fume hood behind a safety shield, and appropriate impermeable gloves, protective clothing, and safety goggles should be worn at all times.
Shipping
UN1953 Compressed gas, toxic, flammable, n.o.s.
Incompatibilities
Heat (at about or above 100 C), shock, friction, concussion, sunlight, or other intense illuminations may cause explosions. Contact with alkali metals; drying agents such as calcium sulfate, or rough edges (such as ground glass) may cause explosions. Diazo compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. This chemical is sensitive to prolonged exposure to heat. This chemical is incompatible with strong oxidizing agents .
Waste Disposal
Decompose chemically with ceric ammonium nitrate under constant agitation and cooling .
Precautions
Diazomethane is attractive as a methylating agent for carboxylic acids and phenols because it reacts quickly and highly efficiently with the production of only N2 as a by-product (Black, 1983). Its natural yellow color is discharged as it reacts, providing automatic indication of reaction progress. However, because diazomethane is highly toxic, it should be generated and used only in a well-functioning fume hood. Because it explodes on contact with some metals or ground glass of any type (joints, stoppers, syringes, stopcocks), it should be handled behind a safety shield, and other personal protective equipment should be used. Because it has a boiling point of ?23°C, it is usually handled in the ethereal solutions in which it is generated. Because it explodes on contact with CaSO4, its solutions or vapors must never be dried with drierite. Despite all of these hazards, it can be worked with safely, provided that appropriate precautions are observed.
Diazomethane Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| REDDY N REDDY PHARMACEUTICALS | +91-9848096759 +91-9848096759 | Hyderabad, India | 1989 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21663 | 55 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 23499 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | China | 35426 | 58 | Inquiry | |
| Portail Substances Chimiques | 10 20 0000 | France | 6027 | 58 | Inquiry |
| SKC Inc. | 724-941-9701 | United States | 1379 | 76 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
Related articles
- Diazomethane - Health Hazard and Toxicity
- Diazomethane has a molecular weight of 42.041 g/mol, and a monoisotopic mass of 42.022 g/mol, which is also its exact mass. Di....
- Sep 12,2019
334-88-3(Diazomethane)Related Search:
1of4
chevron_right




