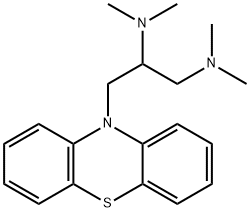
Aminopromazine
- CAS No.
- 58-37-7
- Chemical Name:
- Aminopromazine
- Synonyms
- Proquamezine;aminopromazine;Tetrameprozine;Aminopromazine USP/EP/BP;N1,N1,N2,N2-tetramethyl-3-phenothiazin-10-yl-propane-1,2-diamine;1-N,1-N,2-N,2-N-tetramethyl-3-phenothiazin-10-ylpropane-1,2-diamine;[1-(dimethylaminomethyl)-2-phenothiazin-10-yl-ethyl]-dimethyl-amine;N,N,N',N'-Tetramethyl-3-(10H-phenothiazin-10-yl)-1,2-propanediamine;1,2-Propanediamine, N1,N1,N2,N2-tetramethyl-3-(10H-phenothiazin-10-yl)-
- CBNumber:
- CB4936691
- Molecular Formula:
- C19H25N3S
- Molecular Weight:
- 327.49
- MOL File:
- 58-37-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/4 17:34:40
| NIST Chemistry Reference | 1,2-Propanediamine, n,n,n',n'-tetramethyl-3-(10h-phenothiazin-10-yl)-(58-37-7) |
|---|
Aminopromazine Chemical Properties,Uses,Production
Originator
Jenotone,Coopers
Manufacturing Process
Phenothiazine (20 g) is heated under reflux for 1 hour with sodium amide (5
g) in xylene (80 ml). A solution of 1,3-bis(dimethylamino)-2-chloropropane
(27 g) (prepared by a method analogous to that described in Ingold and
Rothstein J.C.S. 1931, 1676) in xylene (30 ml) is then added over 2 hours.
Heating is continued for a further 1 hour and then the mixture is taken up in
water (270 ml) and hydrochloric acid (d=1.19; 20 ml). The decanted acid
layer is treated with caustic soda (d 1.33; 25 ml) and the base is extracted
with ether (2 x 50 ml) which is then dried over potassium carbonate. On
distillation there is obtained at 218-220°C/0.6 mm Hg a mixture (20 g)
containing a major proportion of 10-[2,3-bis(dimethylamino)-1-
propyl]phenothiazine and a minor proportion of 10-[1,3-bis(dimethylamino)-1-
propyl]phenothiazine.
A mixture of bases (11 g) obtained is dissolved in isopropanol (25 ml). Ether
(25 ml) containing dry hydrogen chloride (2 g) is added, the mixture is left to
crystallise overnight in a refrigerator and the product is filtered off, washed
and dried. There is thus obtained a salt (2.5 g), M.P. 244°C, which is 10-[1,3-
bis(dimethylamino)-1-propyl]phenothiazine hydrochloride.
On evaporation of the mother liquors from the above hydrochloride a residue
is obtained from which is isolated its isomer, 10-[2,3-bis(dimethylamino)-1-
propyl]phenothiazine, the base crystallising from ethanol and melting at 58°C.
The structure of these two isomers has been confirmed by comparison of their
infra-red adsorption spectra with those of related products of known structure.
In practice it is usually used as fumarate.
Therapeutic Function
Spasmolytic
Aminopromazine Preparation Products And Raw materials
Aminopromazine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49392 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8673 | 58 | Inquiry |
| Beijing Four Principles Technology Co., Ltd. | 010-84947198 | China | 72 | 34 | Inquiry |
| Lanospharma Laboratories Co.,Ltd | 13440048448 | China | 6343 | 56 | Inquiry |
| Alfa Chemistry | +1 (201) 478-8534 | United States | 6824 | 0 | Inquiry |
58-37-7(Aminopromazine)Related Search:
1of4
chevron_right




