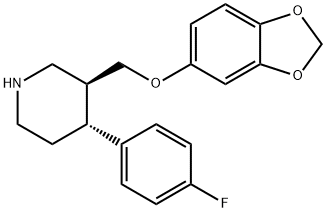
Paroxetine
- CAS No.
- 61869-08-7
- Chemical Name:
- Paroxetine
- Synonyms
- PAXIL;PAROXETIN HCL;AROPAX;FG 7051;BRL 29060;AROPAX 20;Paloxitene;PAROXETINE;PARORETINE HCL;Paroxetine 99%
- CBNumber:
- CB5178021
- Molecular Formula:
- C19H20FNO3
- Molecular Weight:
- 329.37
- MOL File:
- 61869-08-7.mol
- Modify Date:
- 2024/7/2 8:55:11
| Melting point | 114-116°C |
|---|---|
| Boiling point | 451.7±45.0 °C(Predicted) |
| Density | 1.1844 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| form | Solid |
| pka | pKa 9.51 (Uncertain) |
| color | Off-white to light yellow |
| CAS DataBase Reference | 61869-08-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Paroxetine(61869-08-7) |
| EPA Substance Registry System | Paroxetine (61869-08-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| RIDADR | 3249 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
Paroxetine Chemical Properties,Uses,Production
Description
Paroxetine is a new highly selective serotonin reuptake inhibitor, mechanistically similar to fluoxetine, fluvoxamine and sertraline, introduced for the treatment of all types of depressive illnesses including depression associated with anxiety. It is reportedly non-sedating and non-stimulatory and compared to fluoxetine has a shorter duration of action (half-life of 24 hours versus 2 to 3 days). Paroxetine is also being investigated as a treatment for obesity, alcoholism and obsessive-compulsive disorders.
Chemical Properties
White Solid
Uses
A istopically labelled selective serotonin reuptake inhibitor. Used as an antidepressant
Biological Functions
Paroxetine (Paxil) has an elimination half-life of 21 hours and is also highly bound to plasma proteins, so it requires special attention when administered with drugs such as warfarin. Paroxetine is a potent inhibitor of the cytochrome P450 2D6 isoenzyme and can raise the plasma levels of drugs metabolized via this route. Of particular concern are drugs with a narrow therapeutic index, such as TCAs and the type 1C antiarrhythmics flecainide, propafenone, and encainide. Additionally, paroxetine itself is metabolized by this enzyme and inhibits its own metabolism, leading to nonlinear kinetics. Weight gain is higher with paroxetine than with the other SSRIs, and it tends to be more sedating, presumably because of its potential anticholinergic effects. Additionally, patients have had difficulty with abrupt discontinuation with this agent, reporting a flulike syndrome; this symptom can be avoided by tapering the medication.
General Description
In the structure of paroxetine (Paxil), an amino group, protonatedin vivo could H-bond with the–CH2–O– unshared electrons.A β-arylamine–like structure with an extra aryl groupresults. The compound is a very highly selective SERT. Asexpected, it is an effective antidepressant and anxiolytic.
Clinical Use
In vitro binding studies suggest that paroxetine is a more selective and potent inhibitor of 5-HT reuptake than fluoxetine. The drug essentially has no effect on NE or dopamine reuptake, nor does it show affinity for other neuroreceptors. Its onset of action is 1 to 4 weeks.
Paroxetine Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Mylan Laboratories Ltd | +91-4023550543 +91-4030866666 | Telangana, India | 150 | 58 | Inquiry |
| Akums Lifesciences Ltd | +91-1147511000 +91-1147511000 | New Delhi, India | 38 | 58 | Inquiry |
| Wanbury Limited | +91-2271963222 +91-2267942222 | Maharashtra, India | 17 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12558 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANWITA APIS | 58 |
| Ralington Pharma | 58 |
| Mylan Laboratories Ltd | 58 |
| Akums Lifesciences Ltd | 58 |
| Wanbury Limited | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| SynZeal Research Pvt Ltd | 58 |
| Pharmaffiliates Analytics and Synthetics P. Ltd | 58 |
| Hebei Mojin Biotechnology Co., Ltd | 58 |





