Cadmium sulfate
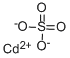
- CAS No.
- 10124-36-4
- Chemical Name:
- Cadmium sulfate
- Synonyms
- ACS, 99 %;CADMIUM SULFATE;CADMIUM SULPHATE;CadMiniuM Sulphate;CadmiumSulphateAcs;cadmiummonosulfate;CADMIUMSULFATE(II);CADMIUM(II)SULPHATE;cadmiumsulfate(1:1);Cadmium sulfate, p.a.
- CBNumber:
- CB5852602
- Molecular Formula:
- CdO4S
- Molecular Weight:
- 208.47
- MOL File:
- 10124-36-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | 41 °C |
|---|---|
| Density | 4.691 |
| vapor pressure | 26.8hPa at 25℃ |
| solubility | water: soluble |
| form | Powder |
| color | White |
| Water Solubility | 1130 G/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,1627 |
| Exposure limits |
ACGIH: TWA 0.01 mg/m3; TWA 0.002 mg/m3 NIOSH: IDLH 9 mg/m3 |
| Stability | Stable. |
| CAS DataBase Reference | 10124-36-4(CAS DataBase Reference) |
| EPA Substance Registry System | Cadmium sulfate (10124-36-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H330-H340-H350-H360FD-H372-H410 | |||||||||
| Precautionary statements | P202-P260-P264-P270-P273-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 45-46-60-61-25-26-48/23/25-50/53 | |||||||||
| Safety Statements | 53-45-60-61 | |||||||||
| RIDADR | UN 2570 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | EV2700000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28332990 | |||||||||
| NFPA 704 |
|
Cadmium sulfate price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 481882 | Cadmium sulfate ≥99.99% trace metals basis | 10124-36-4 | 5G | ₹5250.13 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 481882 | Cadmium sulfate ≥99.99% trace metals basis | 10124-36-4 | 25G | ₹16129.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 383082 | Cadmium sulfate ACS reagent, ≥99.0% | 10124-36-4 | 100G | ₹7588.33 | 2022-06-14 | Buy |
| ALFA India | ALF-011861-22 | Cadmium sulfate, anhydrous, ACS, 99+% | 10124-36-4 | 100g | ₹7559 | 2022-05-26 | Buy |
| ALFA India | ALF-011861-14 | Cadmium sulfate, anhydrous, ACS, 99+% | 10124-36-4 | 25g | ₹2736 | 2022-05-26 | Buy |
Cadmium sulfate Chemical Properties,Uses,Production
Chemical Properties
Cadmium sulfate is a white to colorless, odorless, crystalline substance.
Physical properties
Colorless orthogonal crystal; the hydrates have monoclinic crystal system; density 4.69 g/cm3 (density of mono-, and octahydrates is 3.79 and 3.08 g/cm3, respectively); melts at 1,000°C (octahydrate decomposes at 40°C); soluble in water, insoluble in ethanol.
Uses
Cadmium sulfate (CdS), also called “orange cadmium,” is used to produce phosphors and fluorescent screens. It is also used as a pigment in inks and paints, to color ceramics glazes, in the manufacture of transistors in electronics, photovoltaic cells, and solar cells, and in fireworks.
Preparation
Cadmium sulfate is prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid:
CdO + H2SO4 → CdSO4+ H2
CdO + H2SO4 → CdSO4 + H2O
Cd(OH)2 + H2SO4 → CdSO4+ 2H2O.
General Description
Odorless white solid. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Cadmium sulfate acts as a weakly acidic inorganic salt, which is soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
A confirmed carcinogen.
Health Hazard
Inhalation may cause dryness of throat, coughing, constriction in chest, and headache. Ingestion may cause salivation, vomiting, abdominal pains, or diarrhea. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic cadmium oxide fume may form in fires.
Safety Profile
Confirmed human carcinogen with experimental carcinogenic data. Poison by ingestion, subcutaneous, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Mutation data reported. See also CADMIUM COMPOUNDS and SULFATES. When heated to decomposition it emits very toxic fumes of Cd and SOx.
Potential Exposure
It is used in pigments, electroplating; as a fungicide; and in synthetic and analytical chemistry. Also used in fluorescent screens; as an electrolyte. Incompatibilities: Acts as a weak inorganic acid; neutralizes bases. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, sulfur, selenium, tellurium, zinc
Shipping
UN2570 Cadmium compounds, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Precautions
Cadmium compounds cause more health disorders to occupational workers and persons with pre-existing skin disorders, eye problems, blood disorders, prostate problems, or impaired liver, kidney, or respiratory function. These workers are more susceptible to the effects of cadmium salts. On contact with cadmium, exposed workers should wash the skin and eyes immediately with plenty of water
Cadmium sulfate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| ShanPar Industries Pvt Ltd | +91-2652638973 +91-9909901206 | Gujarat, India | 115 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Indiana Chem- Port | 08048976561 | Vadodara, India | 82 | 58 | Inquiry |
| PK Chlorochem Private Limited | 08048372472Ext 860 | Vadodara, India | 109 | 58 | Inquiry |
| Jyoti Chemicals | 08048372716Ext 693 | Uttar Pradesh, India | 41 | 58 | Inquiry |
| Scientific Syndicate | 08046043822 | Hyderabad, India | 17 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| ShanPar Industries Pvt Ltd | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Alfa Aesar | 58 |
| Central Drug House(P) Ltd. | 58 |
| Indiana Chem- Port | 58 |
| PK Chlorochem Private Limited | 58 |
| Jyoti Chemicals | 58 |
| Scientific Syndicate | 58 |





