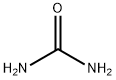
Urea
- CAS No.
- 57-13-6
- Chemical Name:
- Urea
- Synonyms
- CARBAMIDE;Carbamimidic acid;UREA 46;HARNSTOFF;(NH2)2CO;NBK;URE;ISOUREA;aquacare;Pseudourea
- CBNumber:
- CB5853861
- Molecular Formula:
- CH4N2O
- Molecular Weight:
- 60.06
- MOL File:
- 57-13-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/4 14:04:14
| Melting point | 132-135 °C(lit.) |
|---|---|
| Boiling point | 332.48°C (estimate) |
| Density | 1.335 g/mL at 25 °C(lit.) |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index |
n |
| storage temp. | 2-8°C |
| solubility | H2O: 8 M at 20 °C |
| form | powder |
| pka | 0.10(at 25℃) |
| color | white |
| Specific Gravity | 1.335 |
| PH | 8.0-10.0 (20℃, 8M in H2O) |
| Odor | almost odorless |
| Water Solubility | 1080 g/L (20 ºC) |
| λmax |
λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,9867 |
| BRN | 635724 |
| Dielectric constant | 3.5(Ambient) |
| Stability | Substances to be avoided include strong oxidizing agents. Protect from moisture. |
| InChIKey | XSQUKJJJFZCRTK-UHFFFAOYSA-N |
| LogP | -1.660 (est) |
| CAS DataBase Reference | 57-13-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Urea(57-13-6) |
| EPA Substance Registry System | Urea (57-13-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H320 | |||||||||
| Precautionary statements | P264-P337+P313-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 36/37/38-40-38 | |||||||||
| Safety Statements | 26-36-24/25-37 | |||||||||
| RIDADR | Not regulated | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | YR6250000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 31021010 | |||||||||
| Toxicity | LD50 orally in Rabbit: 8471 mg/kg LD50 dermal Rat 8200 mg/kg | |||||||||
| NFPA 704 |
|
Urea price More Price(64)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | U5378 | Urea powder, BioReagent, for molecular biology, suitable for cell culture | 57-13-6 | 100G | ₹4156.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | U7129 | Urea solution 40?% (w/v) in H2O | 57-13-6 | 1VIAL | ₹5986.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | U5378 | Urea powder, BioReagent, for molecular biology, suitable for cell culture | 57-13-6 | 500G | ₹8530.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | U5378 | Urea powder, BioReagent, for molecular biology, suitable for cell culture | 57-13-6 | 1KG | ₹11647.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | U6504 | Urea suitable for electrophoresis | 57-13-6 | 1KG | ₹17233.4 | 2022-06-14 | Buy |
Urea Chemical Properties,Uses,Production
Description
Urea is a stable highly water-soluble compound
of high nitrogen content (47%), with good storage
properties that make it the most commonly used nitrogen
fertilizer. The synthesis process has remained essentially
unchanged since it was first developed by the BASF
Corporation in 1922. In this process, liquid ammonia
is reacted with carbon dioxide to produce ammonium
carbamate, which is then dehydrated to form urea. The
reactions are:
2NH3 + CO2 ===? NH2·CO2·NH4
NH2·CO2·NH4 ===? (NH2)2CO + H2O
Chemical Properties
Urea,CO(HN2)2, also known as carbamide, is a white crystalline powder that has a melting point of l32.7 °C (270 °F). It is a natural product of animal protein metabolism and is the chief nitrogen constituent of urine. Commercially, urea is produced by the reaction of ammonia and carbon dioxide. It is soluble in water, alcohol, and benzene.
Occurrence
The compound was discovered by Hilaire Rouelle in 1773 as a constituent of urine.
History
Urea has the distinction of being the first synthesized organic compound. Until the mid-18th century, scientists believed organic compounds came only from live plants and animals. The first serious blow to the theory of vitalism, which marked the beginning of modern organic chemistry, occurred when Friedrich W?hler (1800 1882) synthesized urea from the two inorganic substances, lead cyanate and ammonium hydroxide: Pb(OCN)2 + 2NH4OH→2(NH2)2CO + Pb(OH)2. W?hler's discoveries on urea occurred while he was studying cyanates; he was attempting to synthesize ammonium cyanate when he discovered crystals of urea in his samples. He first prepared urea in 1824, but he did not identify this product and report his findings until 1828. W?hler's synthesis of urea signaled the birth of organic chemistry.
Uses
Urea is a physiological regulator of nitrogen excretion in mammals; synthesized in the liver as an end-product of protein catabolism and excreted in urine. Also occurs normally in skin. Emollient; diu retic.
Indications
Urea-containing preparations have a softening and moisturizing effect on the stratum
corneum and, at times, may provide good therapy for dry skin and the pruritus
associated with it. They appear to have an antipruritic effect apart from their hydrating
qualities. Urea compounds disrupt the normal hydrogen bonds of epidermal
proteins; therefore, their effect in dry hyperkeratotic diseases such as ichthyosis
vulgaris and psoriasis is not only to make the skin more pliable but also to help
remove adherent scales. Lactic acid also has a softening and moisturizing effect on
the stratum corneum.
Urea 40% ointment may be useful in removing hypertrophic or dystrophic
psoriatic nails. Subsequent topical therapy to the denuded nail bed and proximal
nail fold may result in regrowth of ‘‘normal’’ nails in half of those treated.
Production Methods
Urea is an important industrial compound. The synthesis of urea was discovered in 1870.Commercial production of urea involves the reaction of carbon dioxide and ammonia at highpressure and temperature to produce ammonium carbamate. Ammonium carbamate is thendehydrated to produce urea (Figure 96.1). The reaction uses a molar ratio of ammonia tocarbon dioxide that is approximately 3:1 and is carried out at pressures of approximately 150atmospheres and temperatures of approximately 180°C.
Definition
A white crystalline compound made from ammonia and carbon dioxide. It is used in the manufacture of urea–formaldehyde (methanal) resins. Urea is the end product of metabolism in many animals and is present in urine.
Biological Functions
The use of urea (Ureaphil, Urevert) has declined in recent years owing both to its disagreeable taste and to the increasing use of mannitol for the same purposes. When used to reduce cerebrospinal fluid pressure, urea is generally given by intravenous drip. Because of its potential to expand the extracellular fluid volume, urea is contraindicated in patients with severe impairment of renal, hepatic, or cardiac function or active intracranial bleeding.
General Description
Solid odorless white crystals or pellets. Density 1.335 g /cc. Noncombustible.
Air & Water Reactions
Water soluble.
Reactivity Profile
Urea is a weak base. Reacts with hypochlorites to form nitrogen trichloride which explodes spontaneously in air [J. Am. Chem. Soc. 63:3530-32]. Same is true for phosphorus pentachloride. Urea reacts with azo and diazo compounds to generate toxic gases. Reacts with strong reducing agents to form flammable gases (hydrogen). The heating of improper stoichiometric amounts of Urea and sodium nitrite lead to an explosion. Heated mixtures of oxalic acid and Urea yielded rapid evolution of gases, carbon dioxide, carbon monoxide and ammonia (if hot, can be explosive). Titanium tetrachloride and Urea slowly formed a complex during 6 weeks at 80°C., decomposed violently at 90°C., [Chem. Abs., 1966, 64, 9219b]. Urea ignites spontaneously on stirring with nitrosyl perchlorate, (due to the formation of the diazonium perchlorate). Oxalic acid and Urea react at high temperatures to form toxic and flammable ammonia and carbon monoxide gasses, and inert CO2 gas [Von Bentzinger, R. et al., Praxis Naturwiss. Chem., 1987, 36(8), 41-42].
Health Hazard
May irritate eyes.
Fire Hazard
Behavior in Fire: Melts and decomposes, generating ammonia.
Agricultural Uses
Fertilizer, Fungicide: Used in fertilizers and animal feeds, as a fungicide, in the manufacture of resins and plastics, as a stabilizer in explosives and in medicines, and others. Urea is used to protect against frost and is used in some pesticides as an inert ingredient as a stabilizer, as an inhibitor and as an intensifier for herbicides. Registered for use in EU countries . Registered for use in the U.S.
Trade name
PRESPERSION, 75 UREA®; SUPERCEL 3000®; UREAPHIL®; UREOPHIL®; UREVERT®; VARIOFORM II®
Safety Profile
Moderately toxic by intravenous and subcutaneous routes. Human reproductive effects by intraplacental route: ferthty effects. Experimental reproductive effects. Human mutation data reported. A human skin irritant. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Reacts with sodium hypochlorite or calcium hypochlorite to form the explosive nitrogen trichloride. Incompatible with NaNO2, P2Cl5, nitrosyl perchlorate. Preparation of the 15N-labeled urea is hazardous. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Urea is used in ceramics, cosmetics, paper processing; resins, adhesives, in animal feeds; in the manufacture of isocyanurates; resins, and plastics; as a stabilizer in explosives; in medicines; anticholelithogenic, and others.
Environmental Fate
Terrestrial Fate
Urea is expected to have very high mobility in soil. Urea is not
expected to volatilize from dry soil surfaces based on its vapor
pressure. Various field and laboratory studies have demonstrated
that urea degrades rapidly in most soils. Urea is rapidly hydrolyzed
to ammonium ions through soil urease activity, which
produces volatile gases, that is, ammonia and carbon dioxide.
However, the rate of hydrolysis can be much slower, depending
on the soil type, moisture content, and urea formulation.
Aquatic Fate
Urea is not expected to adsorb to suspended solids and sediments.
Volatilization from water surfaces is not expected. Urea
is rapidly hydrolyzed to ammonia and carbon dioxide in
environmental systems by the extracellular enzyme urease,
which originates from microorganisms and plant roots.
Atmospheric Fate
According to a model of gas/particle partitioning of semivolatile
organic compounds in the atmosphere, urea, which has
a vapor pressure of 1.2×10-5mm Hg at 251°C, will exist in
both the vapor and particulate phases in the ambient atmosphere.
Vapor-phase urea is degraded in the atmosphere by
reaction with photochemically produced hydroxyl radicals; the
half-life for this reaction in air is estimated to be 9.6 days.
Metabolism
The high analysis and good handling properties of urea have made it the leading nitrogen fertilizer, both as a source of nitrogen alone or when compounded with other materials in mixed fertilizers. Although an excellent source of nitrogen, urea can present problems unless properly managed; due to its rapid hydrolysis to ammonia, significant volatilization loss of this may occur if prilled or granular urea is applied to and left on the soil surface without timely incorporation. Mixtures of urea and ammonium nitrate for use in mixed fertilizers are also more highly hygroscopic than ammonium nitrate itself.
Purification Methods
Crystallise urea twice from conductivity water using centrifugal drainage and keeping the temperature below 60o. The crystals are dried under vacuum at 55o for 6hours. Levy and Margouls [J Am Chem Soc 84 1345 1962] prepared a 9M solution in conductivity water (keeping the temperature below 25o) and, after filtering through a medium-porosity glass sinter, added an equal volume of absolute EtOH. The mixture was set aside at -27o for 2-3 days and filtered cold. The precipitate was washed with a small amount of EtOH and dried in air. Crystallisation from 70% EtOH between 40o and -9o has also been used. Ionic impurities such as ammonium isocyanate have been removed by treating the concentrated aqueous solution at 50o with Amberlite MB-1 cation-and anion-exchange resin, and allowing it to crystallise on evaporation. [Benesch et al. J Biol Chem 216 663 1955.] It can also be crystallised from MeOH or EtOH, and is dried under vacuum at room temperature. [Beilstein 3 H 42, 3 I 19, 3 II 35, 3 III 80.]
Incompatibilities
Violent reaction with strong oxidizers, chlorine, permanganates, dichromates, nitrites, inorganic chlorides; chlorites, and perchlorates. Contact with hypochlorites can result in the formation of explosive compounds.
Waste Disposal
Controlled incineration in equipment containing a scrubber or thermal unit to reduce nitrogen oxide emissions.
Urea Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Mumbai, India | 66 | 58 | Inquiry |
| Sudarshan Pharma Industries Limited | +91-2242221111 +91-9320932107 | Maharashtra, India | 37 | 58 | Inquiry |
| Vardhaman P Golechha | 9704422000 | Telangana, India | 294 | 58 | Inquiry |
| Indiana ChemPort | +91-91-2652638667 +91-7574886711 | Gujarat, India | 85 | 58 | Inquiry |
| QUALIKEMS FINE CHEM PVT LTD | +911123618475 | New Delhi, India | 51 | 58 | Inquiry |
| R SCIENTIFIC | +919000280374 | Hyderabad, India | 143 | 58 | Inquiry |
| Yogi Dye Chem Industries | +91-9821098577 +91-9821098577 | Mumbai, India | 124 | 58 | Inquiry |
| Reenold Enterprises | +91-8208173540 +91-8208173540 | Maharashtra, India | 28 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Sudarshan Pharma Industries Limited | 58 |
| Vardhaman P Golechha | 58 |
| Indiana ChemPort | 58 |
| QUALIKEMS FINE CHEM PVT LTD | 58 |
| R SCIENTIFIC | 58 |
| Yogi Dye Chem Industries | 58 |
| Reenold Enterprises | 58 |
| UNILOSA INTERNATINAL PRIVATE LIMITED | 58 |
Related articles
- The Molecular Structure and Properties of Urea
- Urea is a vital organic compound used extensively in various industries due to its unique molecular structure and properties. ....
- Dec 28,2023
- The role of urea in skin care
- Urea is a naturally occurring humectant agent that is part of the water-soluble fraction of the stratum corneum.
- Dec 14,2023
- Urea: Application, toxicity, storage, Preparation
- Urea is a key compound in the metabolism of nitrogen-containing compounds in mammals and is produced naturally in the liver as....
- Apr 23,2023





