Lithium amide
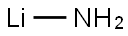
- CAS No.
- 7782-89-0
- Chemical Name:
- Lithium amide
- Synonyms
- Aminolithium;lithamide;Lithiumamid;Lithioamine;Litium amide;ithium amide;LITHIUM AMIDE;lithium azanide;Lithiumamide,95%;Lithium amide (Li(NH2))
- CBNumber:
- CB5854323
- Molecular Formula:
- H2LiN
- Molecular Weight:
- 22.96
- MOL File:
- 7782-89-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/4/23 13:52:06
| Melting point | 373 °C |
|---|---|
| Boiling point | 430 °C |
| Density | 1.178 g/mL at 25 °C(lit.) |
| refractive index | 1.178g/mL |
| storage temp. | water-free area |
| solubility | Slightly soluble in ethanol and liquid ammonia. Insoluble in anhydrous ether, benzene and toluene. |
| form | Powder |
| Specific Gravity | 1.178 |
| color | off-white |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,5522 |
| InChIKey | AFRJJFRNGGLMDW-UHFFFAOYSA-N |
| LogP | 0.23 at 25℃ |
| CAS DataBase Reference | 7782-89-0(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium amide (7782-89-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H261-H314 | |||||||||
| Precautionary statements | P223-P231+P232-P260-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | F,C | |||||||||
| Risk Statements | 14/15-29-34-20/21/22-15-14-11 | |||||||||
| Safety Statements | 26-36/37/39-43-45-7/8-43B-16-3/7/9 | |||||||||
| RIDADR | UN 1390 4.3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | OJ5590000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28530090 | |||||||||
| NFPA 704 |
|
Lithium amide price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 213217 | Lithium amide powder, 95% | 7782-89-0 | 5G | ₹2446.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 213217 | Lithium amide powder, 95% | 7782-89-0 | 5G | ₹2446.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 686050 | Lithium amide hydrogen-storage grade | 7782-89-0 | 10G | ₹5564.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 213217 | Lithium amide powder, 95% | 7782-89-0 | 100G | ₹6971.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 213217 | Lithium amide powder, 95% | 7782-89-0 | 500G | ₹16887 | 2022-06-14 | Buy |
Lithium amide Chemical Properties,Uses,Production
Chemical Properties
white to grey fine powder
Physical properties
Colorless needles; tetragonal structure; density 1.178 g/cm3 at 17.5°C; melts around 375°C; starts to decompose at 320°C; decomposes at 400°C; soluble in cold water; decomposes in hot water; slightly soluble in ethanol and liquid ammonia; insoluble in benzene and ether.
Uses
Lithium amide is used in the preparation of active pharmaceutical ingredients and antioxidants. It acts as a catalyst for polymers, as nucleophiles and as strong bases. It serves as a reagent in the synthesis of antiinflamatory and preoresolving protectin D1, chemotype dipeptidyl peptidase IV inhibitors and sterically congested triarylamines. It finds application in dyes displaying large stokes shifts. In addition to this, it is used as a reagent for cross-coupling of aryl chlorides and amine.
Preparation
Lithium amide, LiNH2 , may be considered the ammonia analogue of lithium hydroxide
in the water system. Lithium amide may be prepared from lithium hydride or lithium
metal and ammonia. Industrial preparations use lithium hydride as a starting material.
The reaction of lithium metal in a stream of ammonia gas at about 400°C may be used
successfully as a preparative method. Lithium may also be reacted with liquid ammonia
in the presence of an iron compound as a catalyst.
Since amide ion is the strongest base which can exist in ammonia, lithium amide is a
very strong base. The compound has a low solubility in liquid ammonia. Lithium amide is
hydrolyzed by water to yield lithium hydroxide and ammonia. It is readily oxidized. For
example, the substance may be oxidized with dinitrogen oxide to yield lithium azide.
Amides of the alkali metals in general must be guarded against air oxidation to prevent the
formation of potentially explosive substances.
General Description
White crystalline powder with an odor of ammonia. Denser than water.
Reactivity Profile
Powdered Lithium amide is highly reactive. A strong base. Reacts to release toxic ammonia gas with water. Forms explosive peroxide on storage.
Health Hazard
Highly toxic: contact with water produces toxic gas, may be fatal if inhaled. Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable and toxic gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
A powerful irritant to skin, eyes, and mucous membranes. Flammable when exposed to heat or flame. Ammonia is liberated and lithmm hydroxide is formed when this compound is exposed to moisture. Reacts violently with water or steam to produce toxic and flammable vapors. Vigorous reaction with oxilzing materials. Exothermic reaction with acid or acid fumes. When heated to decomposition it emits very toxic fumes of LiO, NH3, and NO,. Used in synthesis of drugs, vitamins, steroids, and other organics. See also LITHIUM COMPOUNDS, AMIDES, AMMONIA, and LITHIUM HYDROXIDE.
Purification Methods
Purify it by heating at 400o while NH3 is passed over it in the upper of two crucibles (the upper crucible is perforated). The LiNH2 will drip into the lower crucible through the holes in the upper crucible. The product is cooled in a stream of NH3. Protect it from air and moisture, store it under N2 in a clear glass bottle sealed with paraffin. Store it in small quantities so that all the material is used once the bottle is opened. If the colour of the amide is yellow, it should be destroyed as it is likely to have oxidised and to EXPLODE. On heating above 450o it is decomposed to Li2NH, which is stable up to 750-800o. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 463 1963, Greenlee & Henne Inorg Synth II 135 1953.]
Lithium amide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Nikava Pharmaceutical Industries | +91-9653317212 +91-9653317212 | Mumbai, India | 20 | 58 | Inquiry |
| Organo Metallics | +91-9391016448 +91-9705345345 | Telangana, India | 32 | 58 | Inquiry |
| Alkali Metals Ltd. | 91-40-23445961 | Hyderabad, India | 286 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Mahidhara Chemicals Pvt Ltd | 09949007774 | Telangana, India | 3 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| SL Drugs and Pharmaceuticals Pvt. Ltd. | +91 (40) 6661-1133 | New Delhi, India | 2688 | 50 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Nikava Pharmaceutical Industries | 58 |
| Organo Metallics | 58 |
| Alkali Metals Ltd. | 58 |
| Triveni chemicals | 58 |
| Mahidhara Chemicals Pvt Ltd | 58 |
| CHEMSWORTH | 30 |
| Alfa Aesar | 58 |
| SL Drugs and Pharmaceuticals Pvt. Ltd. | 50 |
| Henan Tianfu Chemical Co.,Ltd. | 55 |
7782-89-0(Lithium amide)Related Search:
1of4
chevron_right




