Nickel fluoride
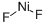
- CAS No.
- 10028-18-9
- Chemical Name:
- Nickel fluoride
- Synonyms
- NiF2;Nickel fluorid;Nickel Flouride;NICKEL FLUORIDE;Nickel difluoride;Nickelous fluoride;NICKEL(II) FLUORIDE;NICKEL FLUORIDE (OUS);Nickel(II) difluoride;Nickel fluoride (NiF2)
- CBNumber:
- CB6714561
- Molecular Formula:
- F2Ni
- Molecular Weight:
- 96.69
- MOL File:
- 10028-18-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/27 17:30:58
| Melting point | 1380℃(lit.) |
|---|---|
| Boiling point | 1498.77°C (estimate) |
| Density | 4.72 g/mL at 25 °C(lit.) |
| vapor pressure | 0.001Pa at 20℃ |
| solubility | diethyl ether: insoluble(lit.) |
| form | Crystalline |
| pka | 0.019[at 20 ℃] |
| Specific Gravity | 4.72 |
| color | Pale yellow |
| Water Solubility | 2.51 g/100 mL |
| Sensitive | Hygroscopic |
| Merck | 14,6508 |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3; TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; IDLH 250 mg/m3; TWA 2.5 mg/m3; TWA 0.015 mg/m3 |
| Stability | hygroscopic |
| CAS DataBase Reference | 10028-18-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Nickel difluoride(10028-18-9) |
| EPA Substance Registry System | Nickel fluoride (NiF2) (10028-18-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H315-H317-H318-H334-H341-H350-H350i-H360D-H372-H410 | |||||||||
| Precautionary statements | P273-P280-P301+P310-P302+P352-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T,Xi,Xn,N | |||||||||
| Risk Statements | 45-36/37/38-42/43-40-68-50/53-48/23-41-38-25-61-49 | |||||||||
| Safety Statements | 53-26-36/37/39-45-36/37-61-60-22 | |||||||||
| RIDADR | 3288 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QR6825000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2826199090 | |||||||||
| Toxicity | LD50 i.v. in mice: 130 mg/kg (Nat. Acad. Sci.) | |||||||||
| NFPA 704 |
|
Nickel fluoride price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 342211 | Nickel(II) fluoride | 10028-18-9 | 10G | ₹11766.78 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 342211 | Nickel(II) fluoride | 10028-18-9 | 50G | ₹27690.35 | 2022-06-14 | Buy |
| ALFA India | ALF-013067-18 | Nickel(II) fluoride, anhydrous, 97% | 10028-18-9 | 50g | ₹27483 | 2022-05-26 | Buy |
| ALFA India | ALF-013067-09 | Nickel(II) fluoride, anhydrous, 97% | 10028-18-9 | 10g | ₹8669 | 2022-05-26 | Buy |
| ottokemi | N 1500 | Nickel fluoride | 10028-18-9 | 100gm | ₹2559 | 2022-05-26 | Buy |
Nickel fluoride Chemical Properties,Uses,Production
Chemical Properties
Green crystalline powder
Uses
Nickel fluoride?comprises the passivating surface that forms on nickel alloys, e.g. monel, which is why such materials are good to store or transport hydrogen fluoride or elemental fluorine.
Preparation
Nickel fluoride tetrahydrate [13940-83-5], NiF2.4H2O, and its anhydrous counterpart, nickel fluoride [10028-18-9], NiF2 , are the only known stable binary compounds of nickel and fluorine. The former is a greenish light yellow crystal or powder prepared by the addition of nickel carbonate to 30-50% aqueous HF solution. The nickel fluoride formed first goes into solution and then precipitates out as the tetrahydrate as the concentration of Nickel fluoride increases and that of HF decreases. When the addition of nickel is complete, the solution and the precipitates are dried at 75-100°C until all the water is expelled. The tetrahydrate has high solubility in aqueous HF, eg, 13.3 wt % in 30% HF. It is slightly soluble in water and insoluble in alcohol and ether.
Anhydrous Nickel fluoride, a light yellow colored powder, is prepared by the action of anhydrous HF on anhydrous NiCl2 , or nickel fluoride tetrahydrate at 300°C. It is also prepared by heating a mixture of NH4.HF2 and NiF2.4H2O. The other methods include the fluorination of metal salts using excess SF4 or using ClF3 at elevated temperatures, or the reaction of NiCO3 and anhydrous HF at 250°C.
Safety Profile
NTP 10th Report on Carcinogens. Reacts violently with potassium. Chronic exposure may cause mottling of teeth, changes in bones. Mutation data reported. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and NICKEL COMPOUNDS.
Nickel fluoride Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| QUALIKEMS FINE CHEM PVT LTD | +911123618475 | New Delhi, India | 51 | 58 | Inquiry |
| SNA HEALTHCARE PVT LTD | +91-8652842416 +91-8652842416 | Mumbai, India | 88 | 58 | Inquiry |
| Jayfluoride PVT LTD | +91-8128987973 +91-6356977002 | Gujarat, India | 27 | 58 | Inquiry |
| Shinde Chemicals Pvt Ltd | +91-9930889810 +91-9930889810 | Maharashtra, India | 15 | 58 | Inquiry |
| Panhas Chemicals Pvt. Ltd. | 91-9586648484 | Gujarat, India | 23 | 58 | Inquiry |
| Shreenivas Chemicals Pvt., Ltd. | 91-22-25641691 | Maharashtra, India | 26 | 58 | Inquiry |
| Jay Intermediates And Chemicals | -2436401 | Gujarat, India | 12 | 58 | Inquiry |
| Qualikems Fine Chem Pvt., Ltd. | 91-265-2841531 | Gujarat, India | 54 | 58 | Inquiry |
| Madras Fluorine Private Ltd. (MFPL) | 91-44-24426830 | Tamil Nadu, India | 8 | 58 | Inquiry |





