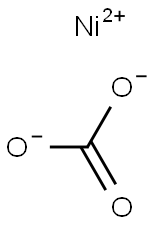
Nickel carbonate
- CAS No.
- 3333-67-3
- Chemical Name:
- Nickel carbonate
- Synonyms
- NICKEL(II) CARBONATE;ci77779;c.i.77779;cino77779;NICKEL CARBONATE;nickle carbonate;Nickel(II)Carbona;nickelmonocarbonate;nickel(2+)carbonate;NICKELOUS CARBONATE
- CBNumber:
- CB1374836
- Molecular Formula:
- CNiO3
- Molecular Weight:
- 118.7
- MOL File:
- 3333-67-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/21 14:26:55
| Melting point | decomposes [CRC10] |
|---|---|
| Density | 4.390 |
| solubility | soluble in dilute acid solutions |
| form | Solid |
| color | Light Green |
| Water Solubility | Soluble in dilute acids. Insoluble in cold water. |
| Solubility Product Constant (Ksp) | pKsp: 6.85 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Stability | Stable. Incompatible with strong acids. |
| CAS DataBase Reference | 3333-67-3(CAS DataBase Reference) |
| EPA Substance Registry System | Nickel carbonate (3333-67-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H317-H332-H334-H341-H350-H360-H372-H400-H410 | |||||||||
| Precautionary statements | P201-P260-P280-P284-P405-P501a | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 22-40-43-50/53 | |||||||||
| Safety Statements | 22-36/37-60-61 | |||||||||
| RIDADR | UN3077 | |||||||||
| RTECS | QR6200000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2836991780 | |||||||||
| NFPA 704 |
|
Nickel carbonate price More Price(2)
Nickel carbonate Chemical Properties,Uses,Production
Chemical Properties
Nickel carbonate is a light green crystalline substance, which is almost insoluble (0.093 g/L) in water (25°C), nonsoluble in hot water, and soluble in acids. Nickel carbonate is available primarily as basic nickel carbonate (NiCO3· 2Ni(OH)2 · 4H2O), which is not soluble in water and soluble in ammonia and dilute acids. In the natural environment, nickel carbonate tetrahydrate can be found as zaratite.

Physical properties
NiCO3: Light green rhombohedral crystals; decomposes on heating; practically insoluble in water, 93 mg/L at 25°C; dissolves in acids.
2NiCO3?3Ni(OH)2?4H2O: Light green crystals or brown powder; decomposes on heating; insoluble in water; decomposes in hot water; soluble in acids and in ammonium salts solutions.
Zaratite: Emerald greed cubic crystals; density 2.6 g/cm3; insoluble in water; soluble in ammonia and dilute acids.
Uses
Nickel carbonate is used to prepare nickel catalysts and several specialty compounds of nickel. It also is used as a neutralizing agent in nickel plating solutions. Other applications are in coloring glass and in the manufacture of ceramic pigments.
Preparation
Anhydrous nickel carbonate is produced as a precipitate when calcium carbonate is heated with a solution of nickel chloride in a sealed tube at 150°C. Alternatively, treating nickel powder with ammonia and carbon dioxide followed by boiling off ammonia yields pure carbonate.
When sodium carbonate is added to a solution of Ni(II) salts, basic nickel carbonate precipitates out in impure form.
Reactions
Nickel carbonate is the starting material for preparing many nickel salts. It reacts with dilute acids evolving carbon dioxide, and upon evaporation of the solution corresponding nickel salts are formed. The nitrate, sulfate and phosphate salts are prepared from carbonate. Similarly, reactions with hydrofluoric, hydrochloric, hydrobromic, or hydriodic acids yield hydrated nickel halides: namely NiF2•4H2O, NiCl2•6H2O, NiBr2•6H2O, and NiI2•6H2O, respectively:
NiCO3 + HCl → NiCl2•6H2O + CO2
NICKEL CARBONATE 611Nickel carbonate decomposes to nickel oxide when strongly ignited:
NiCO3 → NiO + CO2
Nickel carbonate, when dissolved in aqueous thiocyanic acid, yields a yellow brown precipitate of hydrated nickel thiocyanate:
2 NiCO3 + 2HSCN → Ni(SCN)2 + CO2 + H2O
Nickel carbonate forms many double salts, such as, Na2CO3•NiCO3•10H2O with alkali metal carbonates. However, such double carbonates usually are prepared by mixing an alkali metal or ammonium bicarbonate solution with a nickel salt solution, followed by crystallization.
Hazard
Confirmed carcinogen.
Safety Profile
Confirmed human carcinogen. When heated to decomposition it emits toxic vapors of nickel.
Nickel carbonate Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| QUALIKEMS FINE CHEM PVT LTD | +911123618475 | New Delhi, India | 51 | 58 | Inquiry |
| S.V ENTERPRISES | +919322701159 | Mumbai, India | 150 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| Sajan Overseas Pvt Ltd | +91-9825074205 +91-9825074205 | Gujarat, India | 38 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| Cosmic Chemicals | +91-8806467677 +91-9324619871 | Maharashtra, India | 22 | 58 | Inquiry |
| Shriji Chemicals | +91-9822328437 +91-9822328437 | Maharashtra, India | 14 | 58 | Inquiry |
| Eastmen Chemicals | +91-2228727766 +91-2228730294 | Maharashtra, India | 19 | 58 | Inquiry |
| Shinde Chemicals Pvt Ltd | +91-9930889810 +91-9930889810 | Maharashtra, India | 15 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| QUALIKEMS FINE CHEM PVT LTD | 58 |
| S.V ENTERPRISES | 58 |
| Dhara Industries | 58 |
| Sajan Overseas Pvt Ltd | 58 |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | 58 |
| Cosmic Chemicals | 58 |
| Shriji Chemicals | 58 |
| Eastmen Chemicals | 58 |
| Shinde Chemicals Pvt Ltd | 58 |
Related articles
- The Application of Nickel Carbonate as Catalyst
- Nickel carbonate finds applications in sulphamate baths, metal phosphating, electroplating, and ceramic processes. This articl....
- Mar 4,2024
3333-67-3(Nickel carbonate)Related Search:
1of4
chevron_right




