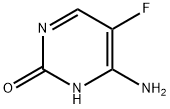
5-Fluorocytosine
- CAS No.
- 2022-85-7
- Chemical Name:
- 5-Fluorocytosine
- Synonyms
- 5-FLUOROCYTOSINE;FLUCYTOSINE;5-FC;ancobon;FLUCYTOSIN;5-FLUCYTOSINE;5-Flurocytosine;4-amino-5-fluoropyrimidin-2(1H)-one;Flurocytosine;5-fluorocytosin
- CBNumber:
- CB6744939
- Molecular Formula:
- C4H4FN3O
- Molecular Weight:
- 129.09
- MOL File:
- 2022-85-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/2/26 19:18:46
| Melting point | 298-300 °C (dec.) (lit.) |
|---|---|
| Density | 1.3990 (estimate) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | 2-8°C |
| solubility | Sparingly soluble in water, slightly soluble in ethanol (96 per cent) |
| form | Crystalline Powder |
| pka | 3.26(at 25℃) |
| color | White to almost white |
| Water Solubility | 1.5g/100mL (25 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,4125 |
| BRN | 127285 |
| BCS Class | 1 |
| Stability | Light Sensitive |
| InChIKey | XRECTZIEBJDKEO-UHFFFAOYSA-N |
| LogP | -1.36 at 22.1℃ and pH6.4-6.9 |
| Dissociation constant | 2.74-10.71 at 21.4℃ |
| CAS DataBase Reference | 2022-85-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361 | |||||||||
| Precautionary statements | P280 | |||||||||
| Hazard Codes | Xn,T,Xi | |||||||||
| Risk Statements | 40-36/37/38-63 | |||||||||
| Safety Statements | 22-24/25-45-36/37-36/37/39-27-26 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | HA6040000 | |||||||||
| F | 10-23 | |||||||||
| Hazard Note | Toxic/Light Sensitive | |||||||||
| HazardClass | IRRITANT, LIGHT SENSITIVE | |||||||||
| HS Code | 29335990 | |||||||||
| Hazardous Substances Data | 2022-85-7(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in mice (mg/kg): >2000 orally and s.c.; 1190 i.p.; 500 i.v. (Grunberg, 1963) | |||||||||
| NFPA 704 |
|
5-Fluorocytosine price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | F7129 | 5-Fluorocytosine nucleoside analog | 2022-85-7 | 1G | ₹24713.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | F7129 | 5-Fluorocytosine nucleoside analog | 2022-85-7 | 5G | ₹119854.4 | 2022-06-14 | Buy |
| TCI Chemicals (India) | F0321 | 5-Fluorocytosine | 2022-85-7 | 1G | ₹2600 | 2022-05-26 | Buy |
| TCI Chemicals (India) | F0321 | 5-Fluorocytosine | 2022-85-7 | 5G | ₹6400 | 2022-05-26 | Buy |
| TCI Chemicals (India) | F0321 | 5-Fluorocytosine | 2022-85-7 | 25G | ₹9700 | 2022-05-26 | Buy |
5-Fluorocytosine Chemical Properties,Uses,Production
Description
5-
Chemical Properties
White Crystalline Solid
Uses
5-Fluorocytosine acts as an antidiabetic, antifungal and antimicrobial agent. It is useful for the treatment of serious infections arises due to susceptible strains of Candida or Cryptococcus neoformans and chromomycosis. Further, it is employed in studies on TMP biosynthesis.
Indications
Flucytosine (Ancobon) is a synthetic, fluorinated pyrimidine that is structurally related to fluorouracil (FU) and floxuridine. It can be fungistatic and fungicidal. Although it is used more frequently in the treatment of systemic infections caused by Candida and Cryptococcus, dermatologic indications may include infections due to chromomycosis, sporotrichosis, Cladosporium, and Sporothrix species. It is generally ineffective against Aspergillus species.
Definition
ChEBI: Flucytosine is an organofluorine compound that is cytosine that is substituted at position 5 by a fluorine. A prodrug for the antifungal 5-fluorouracil, it is used for the treatment of systemic fungal infections. It has a role as a prodrug. It is an organofluorine compound, a pyrimidone, an aminopyrimidine, a nucleoside analogue and a pyrimidine antifungal drug. It is functionally related to a cytosine.
Antimicrobial activity
The spectrum of activity is restricted to Candida spp., Cryptococcus spp. and some fungi causing chromoblastomycosis.
Acquired resistance
About 2–3 of Candida spp. isolates (more in some centers) are resistant before treatment starts, and resistance may develop during treatment. The most common cause of resistance appears to be loss of the enzyme uridine monophosphate pyrophosphorylase.
Pharmaceutical Applications
A synthetic fluorinated pyrimidine available for intravenous infusion or oral administration.
Mechanism of action
Flucytosine (5-flucytosine, 5-FC; Ancoban) is a fluorinated pyrimidine analogue of cytosine that was originally synthesized for possible use as an antineoplastic agent. It is indicated only for the treatment of serious systemic infections caused by susceptible strains of Candida and Cryptococcus spp.The mechanism of action of 5-fluorocytosine (5-FC)has been studied in detail.The drug enters the fungal cell by active transport onATPases that normally transport pyrimidines. Once insidethe cell, 5-fluorocytosine is deaminated in a reaction catalyzedby cytosine deaminase to yield 5-fluorouracil(5-FU). 5-Fluorouracil is the active metabolite of the drug.5-Fluorouracil enters into pathways of both ribonucleotideand deoxyribonucleotide synthesis. The fluororibonucleotidetriphosphates are incorporated into RNA, causingfaulty RNA synthesis. This pathway causes cell death. Inthe deoxyribonucleotide series, 5-fluorodeoxyuridinemonophosphate (F-dUMP) binds to 5,10-methylenetetrahydrofolicacid, interrupting the one-carbon pool substratethat feeds thymidylate synthesis. Hence, DNA synthesisis blocked.
Pharmacology
5-FC is well absorbed orally, with greater than 90% bioavailability. The serum half-life is 3 to 5 hours, with serum levels peaking 4 to 6 hours after a single dose.The drug is widely distributed in body fluids, with cerebrospinal fluid levels 60 to 80% of serum levels.The drug also penetrates well into urine, aqueous humor, and bronchial secretions.Minimal serum protein binding allows more than 90% of each dose to be excreted in the urine; significant dosage reductions are required in the presence of renal impairment. 5-FC can be removed by both hemodialysis and peritoneal dialysis. 5-FC conversion to toxic metabolites may occur in mammalian cells to a limited extent, which accounts for 5-FC toxicity.
Pharmacokinetics
Oral absorption: Complete
Cmax 25 mg/kg 6-hourly oral: 70–80 mg/L after 1–2 h
Plasma half-life: 3–6 h
Volume of distribution: 0.7–1 L/kg
Plasma protein binding c. 12%
Absorption is slower in persons with impaired renal function,
but peak concentrations are higher. Levels in the CSF
are around 75% of the simultaneous serum concentration.
More than 90% of a dose of flucytosine is excreted in the
urine in unchanged form. The serum half-life is much longer
in renal failure, necessitating modification of the dosage regimen:
for patients with a creatinine clearance below 40 mL/
min the dosage interval should be doubled to 12 h; in severe
renal failure the dosage interval should be further increased to
once daily or less, based on frequent serum drug concentration
measurements.
Side effects
Nausea, vomiting, abdominal pain and diarrhea are common.
Serious side effects include myelosuppression and hepatic
toxicity; they occur more frequently when serum concentrations
exceed 100 mg/L.
The nephrotoxic effects of amphotericin B can result in
elevated blood concentrations of flucytosine, and levels of the
latter drug should be monitored when these compounds are
administered together. When 5-FC is prescribed alone to patients with normal renal function, skin rash, epigastric distress, diarrhea, and liver enzyme elevations can occur.
Synthesis
Flucytosine, 5-fluorocytosine (35.4.4), is synthesized from fluorouracil (30.1.3.3). Fluorouracil is reacted with phosphorous oxychloride in dimethylaniline to make 2,4-dichloro-5-fluoropyrimidine (35.4.2), which is reacted with ammonia to make a product substituted with chlorine at the fourth position of the pyrimidine ring—4-amino- 2-chloro-5-fluoropyrimidine (35.4.3). Hydrolysis of the chlorovinyl fragment of this compound in a solution of hydrochloric acid gives the desired flucytosine.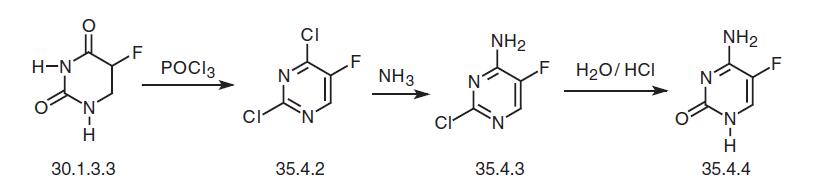
An alternative way of synthesis consists of making flucytosine from a precursor of fluorouracil—5-fluoro-2-methylthiouracil (30.1.3.2) using a somewhat analogous scheme. Treating 5-fluoro-2-methylthiouracil (30.1.3.2) with phosphorous pentachloride gives 4-chloro-5-fluoro-2-methylthiopyrimidine (35.4.5), which upon being reacted with ammonium is transformed into 4-amino-5-fluoro-2-methylthiopyrimidine (35.4.6). Hydrolysis of the methylthiovinyl fragment using concentrated hydrobromic acid gives the desired flucytosine.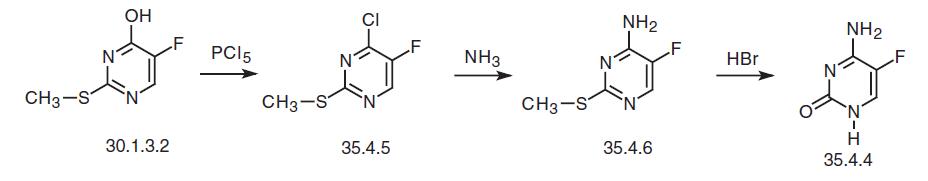
Metabolism
Flucytosine itself is not cytotoxic but, rather, is a pro-drug that is taken up by fungi and metabolized to 5-fluorouracil (5-FU) by fungal cytidine deaminase. Then, 5-FU is converted to 5-fluorodeoxyuridine, which as a thymidylate synthase inhibitor interferes with both protein and RNA biosynthesis. 5-Fluorouracil is cytotoxic and is employed in cancer chemotherapy. Human cells do not contain cytosine deaminase and, therefore, do not convert flucytosine to 5-FU. Some intestinal flora, however, do convert the drug to 5-FU, so human toxicity does result from this metabolism.
5-Fluorocytosine Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SAGAR LIFE SCIENCES PVT LTD | +91-8770359041 +91-8770359041 | Gujarat, India | 79 | 58 | Inquiry |
| Ceyone Life Sciences | +91-8455293199 +91-9348570999 | AndhraPradesh, India | 20 | 58 | Inquiry |
| Aether Industries Limited | +91-261-6603130 +91-2616603130 | Gujarat, India | 145 | 58 | Inquiry |
| Basil Drugs AND Pharmaceuticals Pvt Ltd | +91-2249700250 +91-9619320820 | Mumbai, India | 108 | 58 | Inquiry |
| Solara Active Pharma Sciences Ltd | +91-8046632100 +91-7075706520 | Bangalore, India | 65 | 58 | Inquiry |
| Calyx Chemicals and Pharmaceuticals Ltd | +919819317220 | Mumbai, India | 15 | 58 | Inquiry |
| VGS SYNTHESIS PRIVATE LIMITED | +91-9866975588 +91-9866975588 | Hyderabad, India | 1817 | 58 | Inquiry |
| Apicore Pharmaceuticals Pvt Ltd | +91-2662267177 +91-2662267166 | Gujarat, India | 181 | 58 | Inquiry |
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
Related articles
- Can 5-Fluorocytosine be used to treat cancer?
- Yes. The therapeutic fluoropyrimidines 5-fluorouracil (5-FU) and 5-fluorocytosine (5-FC) have long been used to treat human ca....
- Dec 16,2024
- 5-Fluorocytosine:Introduction and Indication
- 5-Fluorocytosine is clinically licensed for the treatment of cryptococcosis, chromoblastomycosis, and aspergillosis.
- Oct 20,2023
- Capecitabine Intermediate 1
- 5-Fluorocytosine also called Capecitabine Intermediate 1 is an orally active antifungal agent with a very narrow spectrum of a....
- Aug 16,2023
2022-85-7(5-Fluorocytosine)Related Search:
1of4
chevron_right




