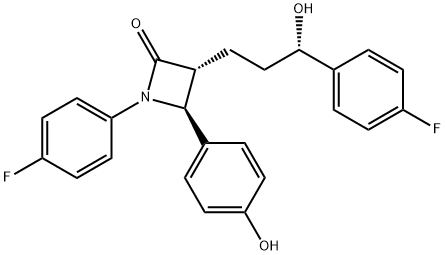
Ezetimibe
- CAS No.
- 163222-33-1
- Chemical Name:
- Ezetimibe
- Synonyms
- ZETIA;EzetiMib;Ezetimide;Ezatimibe;(3R,4S)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)azetidin-2-one;Ezetimibe-001;(3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one;CS-1965;Etimibe;SCH 60969
- CBNumber:
- CB7380787
- Molecular Formula:
- C24H21F2NO3
- Molecular Weight:
- 409.43
- MOL File:
- 163222-33-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/26 17:49:17
| Melting point | 164-166°C |
|---|---|
| alpha | D22 -33.9° (c = 3 in methanol) |
| Boiling point | 654.9±55.0 °C(Predicted) |
| Density | 1.334±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 15 mg/ml) |
| pka | 9.72±0.30(Predicted) |
| form | powder |
| color | White or off-white |
| BCS Class | 2 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| InChI | InChI=1S/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2/t21-,22+,23-/m1/s1 |
| InChIKey | OLNTVTPDXPETLC-XPWALMASSA-N |
| SMILES | N1(C2=CC=C(F)C=C2)[C@H](C2=CC=C(O)C=C2)[C@@H](CC[C@@H](C2=CC=C(F)C=C2)O)C1=O |
| CAS DataBase Reference | 163222-33-1(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Azetidinone, 1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-, (3R,4S)- (163222-33-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H410 |
| Precautionary statements | P273-P391-P501 |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36-24/25 |
| HS Code | 29337900 |
Ezetimibe price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML1629 | Ezetimibe ≥98% (HPLC) | 163222-33-1 | 25MG | ₹13314.75 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1866 | Ezetimibe Pharmaceutical Secondary Standard; Certified Reference Material | 163222-33-1 | 1G | ₹22743.33 | 2022-06-14 | Buy |
Ezetimibe Chemical Properties,Uses,Production
Chemical Properties
White Solid
Uses
Ezetimibe (9) was approved as the first hypolipidemic drug to act by blocking the absorption of dietary cholesterol. This drug was discovered by Schering-Plough and is codeveloped and co-marketed by Merck and Schering-Plough for the treatment of hypercholesterolemia and also two less common forms of hyperlipidemia: homozygous familial hypercholesterolemia and homozygous sitosterolemia.
Definition
ChEBI: Ezetimibe is a beta-lactam that is azetidin-2-one which is substituted at 1, 3, and 4 by p-fluorophenyl, 3-(p-fluorophenyl)-3-hydroxypropyl, and 4-hydroxyphenyl groups, respectively (the 3R,3'S,4S enantiomer). It has a role as an anticholesteremic drug, an antilipemic drug and an antimetabolite. It is a member of azetidines, an organofluorine compound and a beta-lactam.
Biological Functions
Ezetimibe lowers plasma cholesterol levels by inhibiting the absorption of cholesterol at the brush border of the small intestine. Specifically, it has been proposed to bind to a specific transport protein located in the wall of the small intestine, resulting in a reduction of cholesterol transport and absorption. Ezetimibe appears to be selective in its actions in that it does not interfere with the absorption of triglycerides, lipid-soluble vitamins or other nutrients. The decreased absorption of cholesterol eventually leads to enhanced receptor-mediated LDL uptake similar to that seen with bile acid sequestrants and HMGRIs. When used as monotherapy, the decreased absorption of cholesterol causes a compensatory increase in cholesterol biosynthesis. This is similar to that described for bile acid sequestrants and is insufficient to override the overall LDL lowering effects of ezetimibe.
General Description
Ezetimibe, (3R,4S)-1-(4-fluorophenyl)-3-((3S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)-2-azetidinone (Zetia), is an antihyperlipidemicagent that has usefulness in lowering cholesterol levels. Itacts by decreasing cholesterol absorption in the intestine byblocking the absorption of the sterol at the Brush boarder.Specifically, the -lactam binds to the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract that isresponsible for cholesterol absorption. Although it may beused alone, it is marketed as a combination product withsimvastatin under the trade name Vytorin.
Pharmacokinetics
Ezetimibe is administered orally; however, its absolute bioavailability cannot be determined because of its aqueous insolubility and the lack of an injectable formulation. Based on area under the curve values, the oral absorption ranges from 35 to 60%. Mean peak concentrations of the active glucuronidated metabolite are reached within 1 to 2 hours. Both ezetimibe and its glucuronide conjugate are extensively bound (>90%) to plasma proteins. The relative plasma concentrations of ezetimibe and its glucuronide conjugate range from 10 to 20% and from 80 to 90%, respectively. Both compounds have a long half-life of approximately 22 hours. The coadministration of food with ezetimibe has no effect on the extent of absorption.
Clinical Use
Ezetimibe is indicated as monotherapy or in combination with an HMGRI for the reduction of elevated total cholesterol, LDL cholesterol, and apoB in patients with primary (heterozygous familial and nonfamilial) hypercholesterolemia. When used as monotherapy, ezetimibe reduces LDL cholesterol by approximately 18%. When used in combination therapy with an HMGRI, LDL levels are reduced by 25 to 65% depending on the dose of the HMGRI inhibitor. Ezetimibe also is indicated for homozygous familial hypercholesterolemia in combination with either atorvastatin or simvastatin and for homozygous familial sitosterolemia. All indications are for patients who have not responded to diet, exercise, and other nonpharmacological methods.
Side effects
Ezetimibe generally is well tolerated. The most common adverse effects are listed above. Whenever ezetimibe is used in combination with an HMGRI, the incidence of myopathy or rhabdomyolysis does not increase above that seen with HMGRI monotherapy.
Metabolism
Following oral administration, ezetimibe is rapidly and extensively metabolized in the intestinal wall and the liver to its active metabolite, a corresponding phenol glucuronide. This glucuronide is reexcreted in the bile back to its active site. A small amount (<5%) of ezetimibe undergoes oxidation to covert the benzylic hydroxyl group to a ketone; however, ezetimibe does not appear to exert any significant effect on the activity of CYP450 enzymes.
Ezetimibe Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Saptagir Laboratories PVT.LTD. | +91-9059978049 +91-9059978049 | Telangana, India | 31 | 58 | Inquiry |
| SGMR PHARMACEUTICALS PVT LTD | +91-9032001889 +91-9032001889 | Telangana, India | 83 | 58 | Inquiry |
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| SynThink RESEARCH CHEMICALS | +91-8177860948 +91-8177860948 | Mumbai, India | 494 | 58 | Inquiry |
| SEUTIC | +91-8309787199 +91-8309787199 | Hyderabad, India | 124 | 58 | Inquiry |
| TYNDAL LABS PVT LTD | +91-8008166674 +91-8008166674 | AndhraPradesh, India | 95 | 58 | Inquiry |
| SHOBHA LIFE SCIENCES PRIVATE LIMITED | +919121212469 | Hyderabad, India | 31 | 58 | Inquiry |
| Meenaakshi Molecules Pvt Ltd | +91-9121311126 +91-9121311126 | Telangana, India | 45 | 58 | Inquiry |
| Aurobindo Pharma Limited | +914066725000 | Telangana, India | 112 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| Saptagir Laboratories PVT.LTD. | 58 |
| SGMR PHARMACEUTICALS PVT LTD | 58 |
| J S LABS | 58 |
| SynThink RESEARCH CHEMICALS | 58 |
| SEUTIC | 58 |
| TYNDAL LABS PVT LTD | 58 |
| SHOBHA LIFE SCIENCES PRIVATE LIMITED | 58 |
| Meenaakshi Molecules Pvt Ltd | 58 |
| Aurobindo Pharma Limited | 58 |
Related articles
- Ezetimibe-Application in hyperlipidemia
- Ezetimibe is a new cholesterol absorption inhibitor jointly developed by Schering-Plough and Merck. It was approved by the FDA....
- Jan 9,2020





