Celecoxib
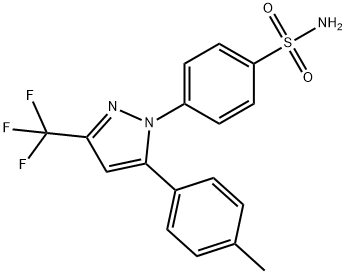
- CAS No.
- 169590-42-5
- Chemical Name:
- Celecoxib
- Synonyms
- CELEBREX;Celocoxib;Celecoxib (200 mg);4-[5-(4-Methylphenyl)-3-trifluoromethyl)-1H-pyrazol-yl]benzenesulfonamide Celecoxib;YM 177;ecoxib;CS-453;Celebra;Celecox;CS-1846
- CBNumber:
- CB7444681
- Molecular Formula:
- C17H14F3N3O2S
- Molecular Weight:
- 381.37
- MOL File:
- 169590-42-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 157-159°C |
|---|---|
| Boiling point | 529.0±60.0 °C(Predicted) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >20mg/mL |
| pka | 9.68±0.10(Predicted) |
| form | powder |
| color | white to off-white |
| Water Solubility | 7mg/L(25 ºC) |
| Merck | 14,1956 |
| BCS Class | 2 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| InChI | InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) |
| InChIKey | RZEKVGVHFLEQIL-UHFFFAOYSA-N |
| SMILES | C1(S(N)(=O)=O)=CC=C(N2C(C3=CC=C(C)C=C3)=CC(C(F)(F)F)=N2)C=C1 |
| CAS DataBase Reference | 169590-42-5(CAS DataBase Reference) |
| EPA Substance Registry System | Benzenesulfonamide, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]- (169590-42-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H360D-H373-H410 |
| Precautionary statements | P202-P260-P273-P280-P308+P313-P391 |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-52-61-60 |
| Safety Statements | 22-24/25-28-37/39 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | DB2944937 |
| HazardClass | IRRITANT |
| HS Code | 29350090 |
Celecoxib price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML3031 | Celecoxib ≥98% (HPLC) | 169590-42-5 | 10MG | ₹9093 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML3031 | Celecoxib ≥98% (HPLC) | 169590-42-5 | 50MG | ₹36707.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1683 | Celecoxib Pharmaceutical Secondary Standard; Certified Reference Material | 169590-42-5 | 1G | ₹9590.95 | 2022-06-14 | Buy |
| TCI Chemicals (India) | C2816 | Celecoxib | 169590-42-5 | 200MG | ₹2600 | 2022-05-26 | Buy |
| TCI Chemicals (India) | C2816 | Celecoxib | 169590-42-5 | 1G | ₹8600 | 2022-05-26 | Buy |
Celecoxib Chemical Properties,Uses,Production
Description
Celecoxib is a nonsteroidal antiinflammatory drug (NSAID) first launched as Celebrex in the US for the treatment of symptoms in patients with rheumatoid arthritis (RA) and osteoarthritis (OA). Celecoxib belongs to a new class of 1, 5-diarylpyrazoles and can be synthesized by heat-promoted heterocyclization of a trifiuoro-l,3-dione with appropriate arylhydrazine. Celecoxib is a highly selective inhibitor of COX-2, the inducible form of cyclooxygenase expressed during inflammatory processes; it does not block the constitutive form COX-1, thus suppressing the gastric and intestinal toxicity of most non-selective NSAIDs. The potency ratio COX1/COX2 on purified human enzymes was about 400. In several in vivo models of acute and chronic inflammation, Celecoxib demonstrated potent antiinflammatory activity without affecting gastric or urinary prostaglandin PGE2. In several clinical studies performed with patients suffering from osteoarthritis or rheumatoid arthritis, Celecoxib was shown to be well tolerated and to relieve pain and inflammation more efficiently compared with other standard NSAIDs; the gastrointestinal safety profile was significantly better than that of other NSAIDs. Interestingly, Celecoxib was approved for another indication in patients with familial adenomatous polyposis (FAP). A six-month clinical trial demonstrated a 28% reduction in the number of colorectal polyps with Celecoxib, compared to a 5% reduction with placebo.
Chemical Properties
White to Pale Yellow Solid
Uses
A selective cyclooxygenase-2 (COX-2) inhibitor. Anti-inflammatory. Used in treatment of familial adenomatous polyposis
Indications
Celecoxib is indicated for the treatment of osteoarthritis and rheumatoid arthritis. Its use is contraindicated in individuals with hypersensitivity to sulfonamides or other NSAIDs. It should be used with caution in persons with hepatic disease. Interactions occur with other drugs that induce CYP2C9 (e.g. rifampin rifampin) or compete for metabolism by this enzyme (e.g. fluconazole, leflunomide). The most common adverse reactions to celecoxib are mild to moderate GI effects such as dyspepsia, diarrhea, and abdominal pain. Serious GI and renal effects have occurred rarely.
General Description
Celecoxib (Celebrex) was the first selective COX-2 inhibitordrug introduced into the market in 1998 for use in thetreatment of RA, OA, acute pain, and menstrual pain. Thereal benefit is that it has caused fewer GI complicationswhen compared with other conventional NSAIDs. It hasalso been approved for reducing the number of adenomatouscolorectal polyps in familial adenomatous polyposis (FAP).Celecoxib is well absorbed and undergoes rapid oxidativemetabolism via CYP2C9 to give its inactive metabolites. Thus, a potential drug interaction exists betweencelecoxib and warfarin because the active isomer ofwarfarin is primarily degraded by CYP2C9.
Biological Activity
Selective cyclooxygenase-2 (COX-2) inhibitor (IC 50 values are 15 and 0.04 μ M for COX-1 and COX-2 respectively). Anti-inflammatory with shorter plasma half-life in vivo than SC 58121 (5-(4-Fluorophenyl)-1-[4-(methylsulfonyl)phenyl]-3-(trifluoromethyl)-1H-pyrazole ). Displays chemopreventive activity in in vivo tumor models.
Pharmacokinetics
Celecoxib is well absorbed from the GI tract, with peak plasma concentrations generally being attained within 3 hours of administration. Peak plasma levels in geriatric patients may be increased, but dosage adjustments in elderly patients generally are not required unless the patient weighs less than 50 kg.
Clinical Use
Celecoxib is currently indicated for the relief of signs and symptoms of osteoarthritis and rheumatoid arthritis and to
reduce the number of adenomatous colorectal polyps in familial adenomatous polyposis as an adjunct to usual care.
Celecoxib is synthesized by condensing 4-methyl-acetophenone and ethyltrifluoroacetate with sodium methoxide and
the resulting butanedione derivative cyclized with 4-hydrazinophenylsulfonamide. It was the first NSAID to be
marketed as a selective COX-2 inhibitor.
Synthesis
Celecoxib is prepared by condensation of 4-methylacetophenone with ethyl trifluoroacetate to give 4,4,4-trifluoro-1-(4- methylphenyl)butane-1,3-dione, which is cyclized with 4-hydrazinophenylsulfonamide.
Metabolism
Celecoxib is excreted in the urine and feces primarily as inactive metabolites, with less than 3% of an administered dose being excreted as unchanged drug. Metabolism occurs primarily in the liver by CYP2C9 and involves hydroxylation of the 4-methyl group to the primary alcohol, which is subsequently oxidized to its corresponding carboxylic acid, the major metabolite (73% of the administered dose). The carboxylic acid is conjugated, to a slight extent, with glucuronic acid to form the corresponding glucuronide. None of the isolated metabolites have been shown to exhibit pharmacological activity as inhibitors of either COX-1 or COX-2. Celecoxib also inhibits CYP2D6; thus, the potential of celecoxib to alter the pharmacokinetic profiles of other drugs inhibited by this isoenzyme exists. Celecoxib, however, does not appear to inhibit other CYP isoforms, such as CYP2C19 or CYP3A4. Other drug interactions related to the metabolic profile of celecoxib have been noted, particularly with other drugs that inhibit CYP2C.
Celecoxib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Cadila Pharmaceuticals Ltd | +91-7069076657 +91-7069076657 | Ahmedabad, India | 54 | 58 | Inquiry |
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| PUNJAB CHEMICALS AND CROP PROTECTION LTD | +91-7508355205 +91-7508355205 | Punjab, India | 69 | 58 | Inquiry |
| Hikal Ltd. | +91-9538444622 +91-8067356100 | Maharashtra, India | 31 | 58 | Inquiry |
| Macleods Pharmaceuticals Limited | +91-2266762800 +91-2266762800 | Maharashtra, India | 116 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| Cadila Pharmaceuticals Ltd | 58 |
| ANWITA APIS | 58 |
| J S LABS | 58 |
| Gonane Pharma | 58 |
| KARPSCHEM LABORATORIES | 58 |
| PUNJAB CHEMICALS AND CROP PROTECTION LTD | 58 |
| Hikal Ltd. | 58 |
| Macleods Pharmaceuticals Limited | 58 |
| Hetero Drugs Limited | 58 |
169590-42-5(Celecoxib)Related Search:
1of4
chevron_right




