Lead dioxide
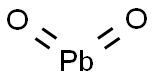
- CAS No.
- 1309-60-0
- Chemical Name:
- Lead dioxide
- Synonyms
- PbO2;Lepro;LP-100;ci77580;leadbrown;c.i.77580;dioxolead;Lead brown;C.I. 77580;Plattnerite
- CBNumber:
- CB9196612
- Molecular Formula:
- O2Pb
- Molecular Weight:
- 239.2
- MOL File:
- 1309-60-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:30
| Melting point | 290 °C |
|---|---|
| Density | 9,38 g/cm3 |
| form | Powder |
| Specific Gravity | 9.38 |
| color | Brown to black |
| Water Solubility | Insoluble |
| Merck | 14,5407 |
| Exposure limits |
ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| CAS DataBase Reference | 1309-60-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Lead dioxide(1309-60-0) |
| EPA Substance Registry System | Lead dioxide (1309-60-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS03,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H302+H332-H360D-H373-H410 | |||||||||
| Precautionary statements | P202-P210-P273-P301+P312-P304+P340+P312-P308+P313 | |||||||||
| Hazard Codes | O,T,N | |||||||||
| Risk Statements | 61-8-20/22-33-50/53-62 | |||||||||
| Safety Statements | 53-45-60-61 | |||||||||
| RIDADR | UN 1872 5.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OG0700000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28249090 | |||||||||
| Toxicity | LD50 i.p. in guinea pigs: 220 mg/kg (Venugopal, Luckey) | |||||||||
| NFPA 704 |
|
Lead dioxide price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 518131 | Lead(IV) oxide 99.998% trace metals basis | 1309-60-0 | 10G | ₹11755.95 | 2022-06-14 | Buy |
| ALFA India | ALF-A12742-36 | Lead(IV) oxide, 97% | 1309-60-0 | 500g | ₹12099 | 2022-05-26 | Buy |
| ALFA India | ALF-A12742-0E | Lead(IV) oxide, 97% | 1309-60-0 | 2500g | ₹45737 | 2022-05-26 | Buy |
| ALFA India | ALF-010729-18 | Lead(IV) oxide, Puratronic™, 99.995% (metals basis) | 1309-60-0 | 50g | ₹46734 | 2022-05-26 | Buy |
| ALFA India | ALF-010729-09 | Lead(IV) oxide, Puratronic™, 99.995% (metals basis) | 1309-60-0 | 10g | ₹11588 | 2022-05-26 | Buy |
Lead dioxide Chemical Properties,Uses,Production
Description
Lead dioxide, PbO2, also plumbic oxide, is an odorless dark-brown crystalline powder which is nearly insoluble in water. It exists in two crystalline forms. The a phase has orthorhombic symmetry, lattice constants a=0.497 nm, b=0.596 nm, c= 0.544 nm, Z=4 (four formula units per unit cell).
Chemical Properties
Lead dioxide is a dark brown crystalline solid or powder.
Physical properties
Red tetragonal crystals or brown powder; density 9.64 g/cm3; decomposes on heating at 290°C; practically insoluble in water; also insoluble in alkalis; moderately soluble in hydrochloric acid and also, in nitric acid-hydrogen peroxide mixture; slowly dissolves in acetic acid.
Occurrence
Lead dioxide occurs in nature as the mineral plattnerite. It is used as an oxidizing agent in manufacturing dyes and intermediates. It also is used as a source of oxygen in matches, pyrotechnics, and explosives. In matches, the oxide is combined with amorphous phosphorus as an ignition surface. It also is used in making lead pigments, liquid polysulfide polymers and rubber substitutes. Lead dioxide electrodes are used in lead storage batteries in which lead dioxide accumulates on positive plates.
Uses
Lead dioxide occurs in nature as the mineral plattnerite. It is used as an oxidizing agent in manufacturing dyes and intermediates. It also is used as a source of oxygen in matches, pyrotechnics, and explosives. In matches, the oxide is combined with amorphous phosphorus as an ignition surface. It also is used in making lead pigments, liquid polysulfide polymers and rubber substitutes. Lead dioxide electrodes are used in lead storage batteries in which lead dioxide accumulates on positive plates.
Preparation
Lead dioxide is produced by oxidizing an alkaline slurry of lead monoxide with chlorine, sodium hypochlorite, or bleaching powder. Alternatively, it is obtained by passing chlorine into a hot aqueous suspension of lead sulfate and magnesium hydroxide. The ionic reaction is:
Pb(OH)3ˉ +ClOˉ → PbO2 + Clˉ+ OHˉ + H2O
It also is produced by electrolysis of acidic solutions of lead salts using a lead or platinum electrode. In such electrolytic process, lead dioxide is deposited on the anode of the cell.
Insoluble powdered lead dioxide also may be obtained when lead tetroxide is heated with nitric acid:
Pb3 O4 + 4HNO3 → 2Pb(N)3)2 + PbO2 + 2H2O
Lead dioxide also can be prepared by fusing lead monoxide with a mixture of sodium nitrate and sodium chlorate.
General Description
Brown, hexagonal crystals. Insoluble in water. Used in matches, explosives, electrodes.
Reactivity Profile
Noncombustible but accelerates the burning of combustible material. Reacts violently with hydrogen sulfide [Bretherick 1979. p. 977-978]. Ignites with hydroxylamine [Mellor 8:291. 1946-47]. Reacts violently with hydrogen peroxide [Mellor 1:937 1946-47], with phenylhydrazine [Mellor 7:637 1946-47], or with sulfuryl chloride [Mellor 10:676. 1946-47]. Reacts with incandescence with sulfur dioxide [Mellor, 1941, Vol. 7, 689]. Explodes when ground with boron or yellow phosphorus [Mellor, 1946, Vol. 5, 17]. Mixtures with sulfur and red phosphorus ignite [Mellor, 1941, Vol. 7, 689]. Reacts vigorously when heated with calcium sulfide, strontium sulfide or barium sulfide [Mellor, 1941, Vol. 3, 745].
Health Hazard
Toxic by ingestion. Inhalation of dust is toxic. Fire may produce irritating, corrosive and/or toxic gases. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. May explode from heat or contamination. Some may burn rapidly. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Potential Exposure
This material is used in electrodes for lead-acid batteries; in matches; explosives, and as a curing agent for polysulfide elastomers
Shipping
UN1872 Lead dioxide, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Incompatibilities
Lead dioxide is a powerful oxidizer. Violent reaction with many compounds, including reducing agents; chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur dioxide, sulfur/friction, sulfuric acid, tungsten, hydrogen trisulfide
Waste Disposal
Conversion to soluble salt, precipitation as sulfide and return to supplier. Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Lead dioxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Nithyasri Chemicals (APURVA CHEMICALS) | +91-2512692130 +91-9323652199 | Maharashtra, India | 75 | 58 | Inquiry |
| L S Chemicals and Pharmaceuticals | +91-9820027610 +91-9820002565 | Maharashtra, India | 10 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3077 | 58 | Inquiry |
| Vishnupriya Chemicals Private Limited | +91-8046041737 | Telangana, India | 220 | 58 | Inquiry |
1309-60-0(Lead dioxide)Related Search:
1of4
chevron_right




