Lead
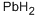
- CAS No.
- 7439-92-1
- Chemical Name:
- Lead
- Synonyms
- Pb;SO;plumbum;plomb;Leadshot;Lead foil;LEAD STANDARD;Lead granular;Lead rod, 5mm (0.2 in.) dia.;Blei
- CBNumber:
- CB9854174
- Molecular Formula:
- Pb
- Molecular Weight:
- 207.2
- MOL File:
- 7439-92-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 327.4 °C (lit.) |
|---|---|
| Boiling point | 1740 °C (lit.) |
| Density | 1.00 g/mL at 20 °C |
| refractive index | 2.881 (632.8 nm) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: soluble |
| form | wire |
| color | Olive-green or red to brown |
| Specific Gravity | 11.288 |
| Odor | Odorless gas |
| PH | 3.8 (20°C in H2O) |
| Flame Color | Blue-white |
| Resistivity | 20.65 μΩ-cm |
| Water Solubility | reacts with hot conc HNO3, boiling conc HCl, H2SO4 [MER06] |
| Merck | 13,5414 |
| Exposure limits | TLV-TWA 0.15 mg/m3 as Pb (ACGIH and MSHA), 0.05 mg (Pb)/m3 (OSHA); 10-h TWA 0.1 mg(inorganic lead)/m3 (NIOSH). |
| Stability | Stable. Incompatible with strong oxidizing agents, potassium, sodium. |
| CAS DataBase Reference | 7439-92-1(CAS DataBase Reference) |
| IARC | 2B (Vol. 23, Sup 7) 1987 |
| EPA Substance Registry System | Lead (7439-92-1) |
| Modulus of Elasticity | 14.0 GPa |
|---|---|
| Poissons Ratio | 0.42 |
| Shear Modulus | 4.90 GPa, calculated |
| Hardness, Vickers | 5.0 |
| Hardness, Brinell | 4.2, Cast |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H360FD-H362-H372 | |||||||||
| Precautionary statements | P202-P260-P263-P264-P270-P308+P313 | |||||||||
| Hazard Codes | T,Xi,Xn,N | |||||||||
| Risk Statements | 61-33-40-48/20-62-36/38-20/22-51/53-50/53-48/20/22-52/53-34-23/24/25 | |||||||||
| Safety Statements | 53-45-61-36/37-36-26-60-36/37/39 | |||||||||
| OEB | D | |||||||||
| OEL | TWA: (8-hour) 0.050 mg/m3 [*Note: The REL also applies to other lead compounds (as Pb) -- see Appendix C.] | |||||||||
| RIDADR | UN 3082 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OF7525000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 78011000 | |||||||||
| Hazardous Substances Data | 7439-92-1(Hazardous Substances Data) | |||||||||
| Toxicity | LDLO oral (pigeon) 160 mg/kg PEL (OSHA) 0.05 mg/m3 PEL (action level) 0.03 mg/m3 TLV-TWA (ACGIH) 0.05 mg/m3 (PEL and TLV apply to lead and inorganic lead compounds) |
|||||||||
| IDLA | 100 mg Pb/m3 | |||||||||
| NFPA 704 |
|
Lead price More Price(80)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 80901 | Lead (impurities) manufactured by MBH Analytical Ltd (83X PR8) | 7439-92-1 | 1EA | ₹49331.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 695912 | Lead shot, 1-3?mm, 99.995% trace metals basis | 7439-92-1 | 25G | ₹18641.85 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 396117 | Lead shot, <2?mm, 99.9% trace metals basis | 7439-92-1 | 2KG | ₹7184.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 265888 | Lead wire, diam. 1.0?mm, 99.99% trace metals basis | 7439-92-1 | 8.9G | ₹7281.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 391352 | Lead powder, ?100?mesh, 99.95% trace metals basis | 7439-92-1 | 100G | ₹22640.3 | 2022-06-14 | Buy |
Lead Chemical Properties,Uses,Production
Description
Lead was one of the earliest metals used by humans, with possible use extending as far back as the seventh millennium BC, and reaching its preindustrial peak usage during the reign of the Roman Empire, around the beginning of the Common Era.
Chemical Properties
Lead is a lustrous silvery metal that tarnishes in the presence of air and becomes a dull bluish gray. The chemical symbol, Pb, is derived from plumbum, the Latin word for waterworks, because of lead’s extensive use in ancient water pipes. Lead has four electrons in its valence shell, but only two ionize readily. The usual oxidation state of lead in inorganic compounds is therefore +2 rather than +4. Lead generally forms stable compounds; the most important ones are lead oxide (PbO) and lead carbonate (PbCO3)2. Four stable lead isotopes exist in nature (208Pb , 206Pb , 207Pb, and 204Pb , in order of abundance). Lead mined from deposits of different geologic eras has entered the environment, so that today there are wide variations and extensive mixture of isotopic ratios of lead in commerce and in the environment. These differences in isotopic ratios may sometimes be used as nonradioactive tracers in environmental and metabolism studies.
Physical properties
Lead is a bluish-white, heavy metallic element with properties that are more metal-like thanthe properties of metalloids or nonmetals. Lead can be found in its native state, meaning thatelemental metallic lead can be found in deposits in the Earth’s crust. However, most lead isfirst mined as galena ore (lead sulfide, PbS). The galena is mixed with lead sulfate, lead sulfide,and lead oxide and is then roasted at a high temperature. The air supply is reduced, followedby an increase in heat and the vaporization of the sulfates and oxides of lead, which are drawnoff as gases. The molten lead is then recovered.
Lead is only slightly soluble in water. However, it is also toxic. This is the reason lead isno longer used to pipe fresh water into homes. It does not react well with acids, with theexception of nitric acid. Lead’s melting point is 327.46°C, its boiling point is 1,740°C, andits density is 11.342 g/cm3.
Isotopes
There are 47 isotopes of lead, four of which are stable. One of these four is Pb-204, which makes up 1.4% of the natural abundance of lead found on Earth. In reality thisisotope is not stable but has a half-life that is so long (1.4×10+17 years), with some of theancient deposits still existing, that it is considered stable. The other three stable isotopes oflead and their proportion to the total natural abundance are as follows: Pb-206 = 24.1%,Pb-207 = 22.1%, and Pb-208 = 52.4%. All the other isotopes are radioactive.
Origin of Name
From the Latin word alumen, or aluminis, meaning “alum,” which is a bitter tasting form of aluminum sulfate or aluminum potassium sulfate.
Occurrence
Lead is the 35th most abundant element on Earth. Although it has been found in its freeelemental metal state, it is usually obtained from a combination of the following ores: galena(PbS), anglesite (PbSO4), cerussite (PbCO3), and minum (Pb3O4). Lead ores are locatedin Europe (Germany, Rumania, and France), Africa, Australia, Mexico, Peru, Bolivia, andCanada. The largest deposits of lead in the United States are in the states of Missouri, Kansas,Oklahoma, Colorado, and Montana.
One of the most famous mining towns is the high-altitude western city of Leadville,Colorado. The boom started with the gold rush of the 1860s, followed by silver mining in the1870s and 1880s. Today, this city is the site of mining operations not only for lead, but alsofor zinc and molybdenum. At the height of its fame, Leadville had a population of almost50,000 people. Today the population is about 2,500.
Lead is commonly obtained by roasting galena (PbS) with carbon in an oxygen-rich environmentto convert sulfide ores to oxides and by then reducing the oxide to metallic lead.Sulfur dioxide gas is produced as a waste product. Large amounts of lead are also recoveredby recycling lead products, such as automobile lead-acid electric storage batteries. About onethirdof all lead used in the United States has been recycled.
Characteristics
Although lead can be found as a metal in the Earth’s crust, it is usually mined and refinedfrom minerals and ores. Lead is one of the most common and familiar metallic elementsknown. Although it is somewhat scarce, found at proportions of 13 ppm, it is still more prevalentthan many other metals. Lead is noncombustible. and it resists corrosion.
When lead, which is very soft, is freshly cut, it has shiny blue-white sheen, which soonoxidizes into its familiar gray color. Lead is extremely malleable and ductile and can be workedinto a variety of shapes. It can be formed into sheets, pipes, buckshot, wires, and powder.Although lead is a poor conductor of electricity, its high density makes it an excellent shieldfor protection from radiation, including X-rays and gamma rays.
Uses
Construction material for tank linings, piping, and other equipment handling corrosive gases and liqs used in the manufacture of sulfuric acid, petroleum refining, halogenation, sulfonation, extraction, condensation; for x-ray and atomic radiation protection; manufacture of tetraethyllead, pigments for paints, and other organic and inorganic lead Compounds; bearing metal and alloys; storage batteries; in ceramics, plastics, and electronic devices; in building construction; in solder and other lead alloys; in the metallurgy of steel and other metals.
Production Methods
The geometric mean soil lead level is 38 mg/kg. Lead
rarely occurs in the elemental state, but exists widely
throughout the world in a number of ores, the most common
of which is the sulfide, galena. The other minerals of commercial
importance are the oxides, carbonate (cerussite), and
the sulfate (anglesite), which are much less common.
Lead also occurs in various uranium and thorium minerals,
arising directly from radioactive decay. Because certain
isotopes are concentrated in lead derivatives from such
sources, both the atomic weight and the density of the
samples vary significantly from normal lead. Lead ores
generally occur in nature in association with silver and
zinc. Other metals commonly occurring with lead ores are
copper, arsenic, antimony, and bismuth. Most of the world production of arsenic, antimony, and bismuth is a result of
their separation from lead ores. Commercial lead ores may
contain as little as 3% lead, but a lead content of 10% is
most common. The ores are concentrated to ≥ 40% lead
content before smelting. A variety of mechanical separation
processes may be employed for the concentration of lead
ores, but the sulfide ores are generally concentrated by
flotation processes.
Definition
lead: Symbol Pb. A heavy dull greysoft ductile metallic element belongingto group 14 (formerly IVB) ofthe periodic table; a.n. 82; r.a.m.207.19; r.d. 11.35; m.p. 327.5°C; b.p.1740°C. The main ore is the sulphidegalena (PbS); other minor sources includeanglesite (PbSO4), cerussite (PbCO3), and litharge (PbO). Themetal is extracted by roasting the oreto give the oxide, followed by reductionwith carbon. Silver is also recoveredfrom the ores. Lead has a varietyof uses including building construction,lead-plate accumulators, bullets,and shot, and is a constituent of suchalloys as solder, pewter, bearing metals,type metals, and fusible alloys.Chemically, it forms compoundswith the +2 and +4 oxidation states,the lead(II) state being the more stable.
General Description
Soft silver-bluish white to gray metal.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
In the presence of carbon, the combination of chlorine trifluoride with aluminum, copper, Lead, magnesium, silver, tin, or zinc results in a violent reaction [Mellor 2, Supp. 1: 1956]. A solution of sodium azide in copper pipe with Lead joints formed copper and Lead azide, both are detonating compounds [Klotz 1973]. Sodium acetylide becomes pyrophoric when mixed with metals like Lead. Mixtures of trioxane with 60% hydrogen peroxide in contact with metallic Lead when heated detonated. Lead containing rubber ignited in a nitric acid atmosphere. Lead is incompatible with strong oxidants such as: ammonium nitrate, chlorine trifluoride, hydrogen peroxide, etc.
Health Hazard
The acute toxicity of lead and inorganic lead compounds is moderate to low.
Symptoms of exposure include decreased appetite, insomnia, headache, muscle and
joint pain, colic, and constipation. Inorganic lead compounds are not significantly
absorbed through the skin.
Chronic exposure to inorganic lead via inhalation or ingestion can result in damage
to the peripheral and central nervous system, anemia, and chronic kidney disease.
Lead can accumulate in the soft tissues and bones, with the highest accumulation in
the liver and kidneys, and elimination is slow. Lead has shown developmental and
reproductive toxicity in both male and female animals and humans. Lead is listed by
IARC in Group 2B ("possible human carcinogen") and by NTP as "reasonably
anticipated to be a carcinogen," but is not considered to be a "select carcinogen"
under the criteria of the OSHA Laboratory Standard.
Fire Hazard
Flash point data for Lead are not available, however, Lead is probably non-combustible.
Flammability and Explosibility
Lead powder is combustible when exposed to heat or flame.
Industrial uses
Not only is lead the most impervious of all common metals to x-rays and gamma radiation, it also resists attack by many corrosive chemicals, most types of soil, and marine and industrial environments. Although lead is one of the heaviest metals, only a few applications are based primarily on its high density. The main reasons for using lead often include low melting temperature, ease of casting and forming, good sound and vibration absorption, and ease of salvaging from scrap.
With its high internal damping characteristics, lead is one of the most efficient sound attenuators for industrial, commercial, and residential applications. Sheet lead, lead-loaded vinyls, lead composites, and lead-containing laminates are used to reduce machinery noise. Lead sheet with asbestos or rubber sandwich pads are commonly used in vibration control.
Carcinogenicity
Lead and lead compounds are reasonably anticipated to be human carcinogens based on limited evidence of carcinogenicity from studies in humans and sufficient evidence of carcinogenicity from studiesin experimental animals.
storage
work with lead dust, molten lead, and lead salts capable of forming dusts should be conducted in a fume hood to prevent exposure by inhalation.
Incompatibilities
Violent reactions of lead with sodium azide, zirconium, sodium acetylide, and chlorine trifluoride have been reported. Reactivity of lead compounds varies depending on structure.
Waste Disposal
Excess lead and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. For more information on disposal procedures, see Chapter 7 of this volume.
Lead Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Gharda Chemicals Ltd | +919820134485 | Maharashtra, India | 32 | 58 | Inquiry |
| Aryavart Chemicals Pvt Ltd | +91-9920698991 +91-9920698991 | Maharashtra, India | 6 | 58 | Inquiry |
| Pondy Oxides And Chemicals Ltd. POCL | +91-4442965454 +91-4442965454 | Tamil Nadu, India | 2 | 58 | Inquiry |
| Hindustan Zinc | +91-2946604002 +91-2946604000 | Rajasthan, India | 2 | 58 | Inquiry |
| Mittal Pigments Pvt Ltd | +91-9829626774 +91-9829626774 | Rajasthan, India | 7 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6157 | 58 | Inquiry |
| Aritech Chemazone Private Limited | +91-9034345475 | Punjab, India | 684 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3075 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6088 | 58 | Inquiry |
Related articles
- How are Lead Minerals Distributed?
- Many lead minerals are relatively light and, in Earth’s history, have stayed in the crust instead of sinking deeper into the E....
- May 31,2024
- Lead-Hazard and Toxicity
- Lead is a bluish-white, heavy metallic element with properties that are more metal-like than the properties of metal loids or ....
- Sep 9,2019





