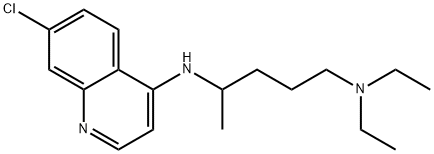
CHLOROQUINE
|
- ₹0
- Product name: CHLOROQUINE
- CAS: 54-05-7
- MF: C18H26ClN3
- MW: 319.87
- EINECS:200-191-2
- MDL Number:MFCD00024009
- Synonyms:sn7618 ;Solprina;Sopaquin;Tanakan;Tresochin;Trochin;W 7618;w7618
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :87°
Boiling point :475.41°C (rough estimate)
Density :1.0500 (rough estimate)
refractive index :1.6010 (estimate)
storage temp. :2-8°C(protect from light)
solubility :Chloroform (Slightly), Methanol (Slightly)
form :Solid
pka :pKa 8.4(H2O t = 20) (Uncertain)
color :White to Light Brown
Stability :Stable, but light sensitive. Incompatible with strong oxidizing agents.
IARC :3 (Vol. 13, Sup 7) 1987
EPA Substance Registry System :1,4-Pentanediamine, N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl- (54-05-7)
Boiling point :475.41°C (rough estimate)
Density :1.0500 (rough estimate)
refractive index :1.6010 (estimate)
storage temp. :2-8°C(protect from light)
solubility :Chloroform (Slightly), Methanol (Slightly)
form :Solid
pka :pKa 8.4(H2O t = 20) (Uncertain)
color :White to Light Brown
Stability :Stable, but light sensitive. Incompatible with strong oxidizing agents.
IARC :3 (Vol. 13, Sup 7) 1987
EPA Substance Registry System :1,4-Pentanediamine, N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl- (54-05-7)
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Chloroquine is the most effective of the hundreds of 4-aminoquinolines synthesized and tested during World War II as potential antimalarials. Structure–activity relationships demonstrated that the chloro at the 8-position increased activity, whereas alkylation at C-3 and C-8 diminished activity. The replacement of one of its N-ethyl groups with an hydroxyethyl produced hydroxychloroquine, a compound with reduced toxicity that is rarely used today except in cases of rheumatoid arthritis.More related product prices
Mepacrine hydrochloride Chloroquine diphosphate Chloroquine sulfate 4,7-DichloroquinolineRelated product price
- Hydroxychloroquine sulfate
₹5174.35-47857.33 - Chloroquine diphosphate
₹4400-48247.03 - 4213-45-0
₹9580.13-64874.23






