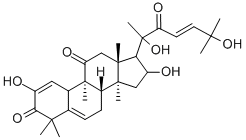
CUCURBITACIN I
|
- ₹25146.48
- Product name: CUCURBITACIN I
- CAS: 2222-07-3
- MF: C30H42O7
- MW: 514.65
- EINECS:218-736-8
- MDL Number:MFCD09971044
- Synonyms:ELATERICIN B;ELATERIN B;CUCURBITACIN I WITH HPLC;Cucurbitacin I hydrate;Elatericin B, JSI-124, NSC 521777, 2,16α,20,25-Tetrahydroxy-9-methyl-19-Nor-9β,10α-lanosta-1,5,23-triene-3,11,22-trione;(9β,10α,23E)-2,16α,20,25-Tetrahydroxy-9-methyl-19-norlanosta-1,5,23-triene-3,11,22-trione;Ibamarin;2,16a,20,25-Tetrahydroxy-9b-methyl-10a-19-norlanosta-1,5,23(E)-triene-3,11,22-trione
1 prices
Selected condition:
Brand
- Sigma-Aldrich(India)
Package
- 1MG
- ManufacturerSigma-Aldrich(India)
- Product numberC4493
- Product descriptionCucurbitacin I hydrate ≥95% (HPLC), solid
- Packaging1MG
- Price₹25146.48
- Updated2022-06-14
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | C4493 | Cucurbitacin I hydrate ≥95% (HPLC), solid | 1MG | ₹25146.48 | 2022-06-14 | Buy |
Properties
Melting point :148-150°C
Boiling point :698.3±55.0 °C(Predicted)
Density :1.26±0.1 g/cm3(Predicted)
storage temp. :-20°C
solubility :≥22.45 mg/mL in DMSO; insoluble in EtOH; ≥51.2 mg/mL in H2O with ultrasonic
pka :8.51±0.70(Predicted)
form :solid
color :white to off-white
LogP :2.330 (est)
Boiling point :698.3±55.0 °C(Predicted)
Density :1.26±0.1 g/cm3(Predicted)
storage temp. :-20°C
solubility :≥22.45 mg/mL in DMSO; insoluble in EtOH; ≥51.2 mg/mL in H2O with ultrasonic
pka :8.51±0.70(Predicted)
form :solid
color :white to off-white
LogP :2.330 (est)
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Cucurbitacin I is triterpenoid compound that acts as a potent inhibitor of the STAT3/JAK signaling pathway. It specifically suppresses levels of tyrosine phosphorylated STAT3 in v-Src-transformed NIH 3T3 cells and in A549 cells (IC50 = 500 nM) resulting in inhibition of STAT3 DNA binding and reduced STAT3-mediated gene transcription. It also suppresses JAK2 phosphorylation but does not affect Src, ERK, JNK or Akt. In nude mice, cucurbitacin I(1 mg/kg/day) suppressed growth of various tumors expressing constitutively active STAT3. It promotes the differentiation of dendritic cells and macrophages and enhances the effect of cancer immunotherapy. Cucubitacin I (1 μM for 2 hours) reduced clonogenicity of nasopharyngeal carcinoma cells in vitro and suppresses tumor growth in mice (1.3 mg/kg).
More related product prices
2,6-Dimethyl-5-heptenal Menthone 1,2-Cyclohexanedione 2-AllylcyclohexanoneRelated product price
- 2,6-Dimethyl-5-heptenal
₹3900-144513.75 - CUCURBITACIN I
₹25146.48 - Menthone
₹6332.63-25049.05






