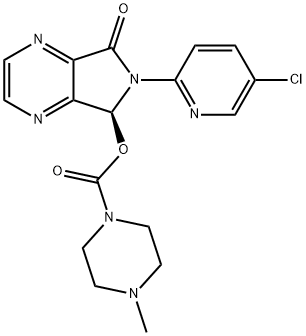
Eszopiclone
|
- ₹0
- Product name: Eszopiclone
- CAS: 138729-47-2
- MF: C17H17ClN6O3
- MW: 388.81
- EINECS:202-303-5
- MDL Number:MFCD03700720
- Synonyms:ESOPICLONE;ESZOPICLONE;(S)-(+)-ZOPICLONE;[(9S)-8-(5-Chloropyridin-2-yl)-7-oxo-2,5,8-triazabicyclo[4.3.0]nona-1,3,5-trien-9-yl]4-methylpiperazine-1-carboxylate;Lunesta;ESZOPICLONE CIV;1-Piperazinecarboxylic acid, 4-methyl-, (5S)-6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester (9CI);1-Piperazinecarboxylic acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester, (S)-
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :202-204°C
alpha :D20 +135 ±3° (c = 1.0 in acetone)
Boiling point :580.7±50.0 °C(Predicted)
Density :1.54±0.1 g/cm3(Predicted)
storage temp. :-20°C Freezer
solubility :Chloroform (Slightly, Heated), Methanol (Slightly, Heated)
form :Solid
pka :6.70±0.10(Predicted)
color :White to Light Beige
InChI :InChI=1S/C17H17ClN6O3/c1-22-6-8-23(9-7-22)17(26)27-16-14-13(19-4-5-20-14)15(25)24(16)12-3-2-11(18)10-21-12/h2-5,10,16H,6-9H2,1H3/t16-/m0/s1
InChIKey :GBBSUAFBMRNDJC-INIZCTEOSA-N
SMILES :N1(C(O[C@H]2C3=NC=CN=C3C(=O)N2C2=NC=C(Cl)C=C2)=O)CCN(C)CC1
CAS DataBase Reference :138729-47-2(CAS DataBase Reference)
alpha :D20 +135 ±3° (c = 1.0 in acetone)
Boiling point :580.7±50.0 °C(Predicted)
Density :1.54±0.1 g/cm3(Predicted)
storage temp. :-20°C Freezer
solubility :Chloroform (Slightly, Heated), Methanol (Slightly, Heated)
form :Solid
pka :6.70±0.10(Predicted)
color :White to Light Beige
InChI :InChI=1S/C17H17ClN6O3/c1-22-6-8-23(9-7-22)17(26)27-16-14-13(19-4-5-20-14)15(25)24(16)12-3-2-11(18)10-21-12/h2-5,10,16H,6-9H2,1H3/t16-/m0/s1
InChIKey :GBBSUAFBMRNDJC-INIZCTEOSA-N
SMILES :N1(C(O[C@H]2C3=NC=CN=C3C(=O)N2C2=NC=C(Cl)C=C2)=O)CCN(C)CC1
CAS DataBase Reference :138729-47-2(CAS DataBase Reference)
Safety Information
| Symbol(GHS): |
 
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | |||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
Eszopiclone is a non-benzodiazepine hypnotic agent indicated for the treatment of insomnia to induce sleep and for sleep maintenance. It has similar pharmacokinetic and pharmacodynamic parameters as the previously marketed non-benzodiazepine hypnotics zolpidem and zaleplon. However, unlike its predecessors, eszopiclone is not restricted to short-term treatment of insomnia. Clinical studies of up to 6 months of use show that patients do not develop tolerance to its effect. Eszopiclone is the (S)-enantiomer of zopiclone, which has been marketed as the racemic mixture in Europe for almost 20 years. These agents belong to the cyclopyrrolone class of drugs that act as agonists at the type A GABA receptor. Eszopiclone has approximately 50-fold higher binding affinity than its antipode (R)-zopiclone for GABA-A receptor (IC50=21 and 1130 nM, respectively). In addition, the two enantiomers exhibit significant differences in their pharmacokinetic parameters and in vivo efficacy. In healthy volunteers, eszopiclone has 2-fold higher Cmax and 2-fold greater elimination half-life than the (R)-enantiomer.The two most frequent adverse events associated with eszopiclone treatment are unpleasant taste and headache. Other less frequent side effects include somnolence, dry mouth, and nausea.More related product prices
6-(5-Chloro-2-pyridyl)-6,7-dihydro-7-hydroxy-5H-pyrrolo[3,4-b]pyrazin-5-one SEP 174559 Hydrochloride Ozagrel Pramipexole dihydrochloride (S)-(+)-2-Chlorophenylglycine methyl ester ZOPICLONE N-OXIDE 6-(5-Chloro-2-pyridyl)-5H-pyrrolo[3,4-b]pyrazine-5,7(6H)-dione Pyrrole Zopiclone EszopicloneRelated product price
- Pramipexole dihydrochloride
₹9428.58-38461.23 - ZOPICLONE N-OXIDE
₹18101.3 - Pyrrole
₹900-108498.98
Suppliers and manufacturers
Dr. Reddy's Laboratories Ltd
Apotex Pharmachem India Pvt Ltd
Glenmark Pharmaceuticals Limited
Viyash Life Sciences Pvt Ltd
Calyx Chemicals and Pharmaceuticals Ltd
Hetero Drugs Limited
Macleods Pharmaceuticals Limited
Centaur Pharmaceuticals Pvt Ltd
Orchid Pharma Ltd. (Formerly Known as Orchid Chemicals and Pharmaceuticals Ltd.)
Ralington Pharma






