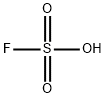
FLUOROSULFONIC ACID
|
- ₹16908.65 - ₹44360.85
- Product name: FLUOROSULFONIC ACID
- CAS: 7789-21-1
- MF: FHO3S
- MW: 100.07
- EINECS:232-149-4
- MDL Number:MFCD00066174
- Synonyms:fluorosulfuricacid(hso3f) ;fluosulfuricacid ;monofluorosulfuricacid ;FLUOSULFONIC ACID;FLUOROSULFONIC ACID;Fluorosulforic Acid;fluorosulphuric acid;FLUOROSULFONIC ACID, TRIPLE DISTILLED
2 prices
Selected condition:
Brand
- Sigma-Aldrich(India)
Package
- 25ML
- 100ML
- ManufacturerSigma-Aldrich(India)
- Product number236535
- Product descriptionFluorosulfonic acid purified by triple-distillation
- Packaging100ML
- Price₹44360.85
- Updated2022-06-14
- Buy
- ManufacturerSigma-Aldrich(India)
- Product number236535
- Product descriptionFluorosulfonic acid purified by triple-distillation
- Packaging25ML
- Price₹16908.65
- Updated2022-06-14
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 236535 | Fluorosulfonic acid purified by triple-distillation | 100ML | ₹44360.85 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 236535 | Fluorosulfonic acid purified by triple-distillation | 25ML | ₹16908.65 | 2022-06-14 | Buy |
Properties
Melting point :−87.3 °C(lit.)
Boiling point :165.5 °C(lit.)
Density :1.726 g/mL at 25 °C(lit.)
vapor density :3.5 (vs air)
vapor pressure :2.5 mm Hg ( 25 °C)
storage temp. :2-8°C
solubility :reacts with H2O
pka :-6.29±0.15(Predicted)
form :colorless liquid
color :colorless
Water Solubility :hydrolyzes violently in H2O; reddish?brown color in acetone [MER06]
Merck :13,4206
Stability :Stable. May corrode glass. Incompatible with many metals, bases.
CAS DataBase Reference :7789-21-1(CAS DataBase Reference)
EPA Substance Registry System :Fluorosulfuric acid (7789-21-1)
Boiling point :165.5 °C(lit.)
Density :1.726 g/mL at 25 °C(lit.)
vapor density :3.5 (vs air)
vapor pressure :2.5 mm Hg ( 25 °C)
storage temp. :2-8°C
solubility :reacts with H2O
pka :-6.29±0.15(Predicted)
form :colorless liquid
color :colorless
Water Solubility :hydrolyzes violently in H2O; reddish?brown color in acetone [MER06]
Merck :13,4206
Stability :Stable. May corrode glass. Incompatible with many metals, bases.
CAS DataBase Reference :7789-21-1(CAS DataBase Reference)
EPA Substance Registry System :Fluorosulfuric acid (7789-21-1)
Safety Information
| Symbol(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
Fluorosulphuric acid (FSO3H) is a free-flowing colourless fuming liquid. It is soluble in polar organic solvents such as acetic acid, ethyl acetate, and nitrobenzene but poorly soluble in nonpolar solvents such as alkanes. FSO3H is corrosive to metals and body tissues, corrodes glass, and is incompatible with many metals and bases. With its strong acidity, FSO3H dissolves almost all organic compounds that are even weak proton acceptors. FSO3H hydrolyses slowly to HF and sulphuric acid (H2SO4). The related triflic acid (CF3SO3H) retains the high acidity of FSO3H but is more hydrolytically stable. FSO3H is one of the strongest known simple Bronsted acids. FSO3H is useful for regenerating mixtures of HF and H2SO4 for etching lead glass. FSO3H isomerises alkanes and the alkylation of hydrocarbons with alkenes, although it is unclear if such applications are of commercial importance. FSO3H is used as a catalyst in organic synthesis and in electroplating and as a fluorinating agent and as a laboratory fluorinating agent. FSO3H has been reported as a highly toxic and corrosive chemical substance. FSO3H is non-combustible but enhances combustion of other substances. It gives off irritating or toxic fumes (or gases) in a fire. FSO3H hydrolyses to release HF. Addition of water to FSO3H causes very violent reactions similar to the addition of water to H2SO4. Addition of FSO3H to water is a much more violent process than addition of H2SO4. The combination of FSO3H and others is categorised as ‘magic acids and super acids’. Very short contact and the fumes of FSO3H cause severe painful burns.Related product price
- FLUOROSULFONIC ACID
₹16908.65-44360.85 - POTASSIUM FLUOROSULFATE
₹3215.03 - TATM
₹2359.85-15219.95






