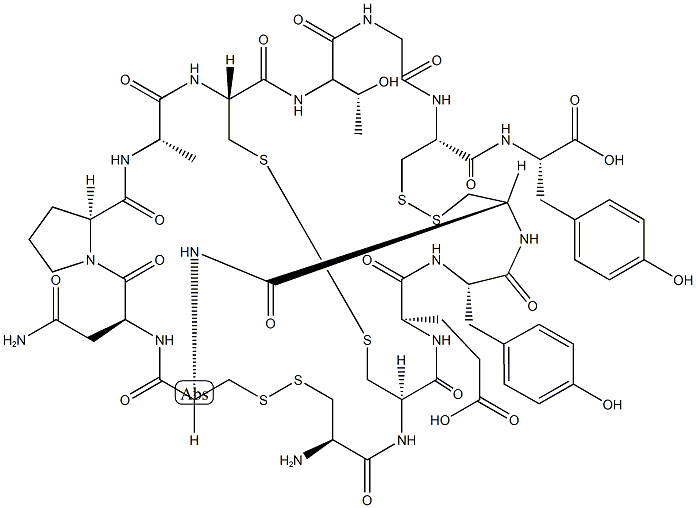
Linaclotide
|
- ₹0
- Product name: Linaclotide
- CAS: 851199-59-2
- MF: C59H79N15O21S6
- MW: 1526.74
- EINECS:251-228-4
- MDL Number:MFCD20526656
- Synonyms:Linaclotide;Linaelotide Acetate;Linaelotide;CY-14;Liclotide;Argpessin;L-Tyrosine, L-cysteinyl-L-cysteinyl-L-α-glutamyl-L-tyrosyl-L-cysteinyl-L-cysteinyl-L-asparaginyl-L-prolyl-L-alanyl-L-cysteinyl-L-threonylglycyl-L-cysteinyl-, cyclic (1→6),(2→10),(5→13)-tris(disulfide);Linaclotide USP/EP/BP
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :231-235°C (dec.)
Boiling point :2045.0±65.0 °C(Predicted)
Density :1.60
storage temp. :Hygroscopic, Refrigerator, under inert atmosphere
solubility :DMSO (Slightly, Heated), Methanol ((Slightly, Heated)), Water (Slightly, Heated)
pka :3.05±0.10(Predicted)
form :Solid
color :White
Stability :Hygroscopic
InChIKey :KXGCNMMJRFDFNR-VRMHCMCOSA-N
Boiling point :2045.0±65.0 °C(Predicted)
Density :1.60
storage temp. :Hygroscopic, Refrigerator, under inert atmosphere
solubility :DMSO (Slightly, Heated), Methanol ((Slightly, Heated)), Water (Slightly, Heated)
pka :3.05±0.10(Predicted)
form :Solid
color :White
Stability :Hygroscopic
InChIKey :KXGCNMMJRFDFNR-VRMHCMCOSA-N
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
In August 2012, the US FDA approved linaclotide (also referred to as MD-1100), a first-in-class, orally administered 14-amino acid peptide as a therapy for patients suffering from chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). Linaclotide and its active metabolite MM-419447, which results from the cleavage of the C-terminal tyrosine residue by carboxypeptidase A, mimic the actions of the endogenous intestinal peptides guanylin (15 amino acids) and uroguanylin (16 amino acids) by activating guanylyl cyclase C (GC-C) on the intestinal epithelium. Activation of GC-C leads to increased intra- and extracellular levels of cGMP and activation of the CFTR ion channel, resulting in increased levels of HCO3-, Cl-, and water in the intestinal lumen and accelerated gastrointestinal transit. Based on an in vitro assaymeasuring the accumulation of cGMP in T84 cell exposed to an agonist, the EC50 of linaclotide at pH 7.0 was 99±17.5 nM. In preclinical studies in mice using the transit of activated charcoal as ameasure of efficacy, linaclotide at 100 μg/kg significantly accelerated transit compared to wild-type mice treated with charcoal only or GC-Cnullmice treatedwith and without linaclotide.118 Efficacy was also seen in rats treatedwith linaclotide atdoses of 5, 10, and 20 μg/kg. Linaclotide has been synthesized using conventional solid-phase peptide technology.More related product prices
Octreotide acetate Liraglutide Ziconotide acetate Bivalirudin Bisacodyl Sofosbuvir Rifaximin 4-Amino-5-chloro-2-ethoxy-N-((4-(4-fluorobenzyl)-2-morpholinyl)methyl)benzamide Pinaverium bromide Leuprorelin Somatostatin Elcatonin Tenofovir Zafirlukast Triptorelin Leuprorelin acetate NafarelinRelated product price
- Octreotide acetate
₹28318.2-41232.43 - Ziconotide acetate
₹28199.13-101527.68 - Bivalirudin
₹10078.08-40269






