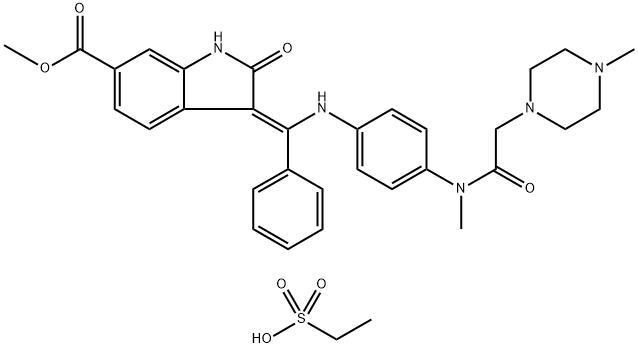
Nintedanib esylate
|
- ₹0
- Product name: Nintedanib esylate
- CAS: 656247-18-6
- MF: C33H39N5O7S
- MW: 649.76
- EINECS:
- MDL Number:MFCD26142360
- Synonyms:(3Z)-2,3-Dihydro-3-[[[4-[methyl[2-(4-methyl-1-piperazinyl)acetyl]amino]phenyl]amino]phenylmethylene]-2-oxo-1H-indole-6-carboxylic acid methyl ester ethanesulfonate ;BIBF 1120 esylate;BIBF-1120 esylate;BIBF1120/nintedanib ethanesulfonate salt;Trinidad Neeb esylate;Intedanib ethanesulfonate;Nintedanib ethanesulfonate salt;Trinidad Neeb ethanesulfonate
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :>233°C (dec.)
storage temp. :-20°C Freezer
solubility :DMSO (Slightly), Methanol (Slightly)
form :Yellow solid.
color :Light Yellow to Yellow
storage temp. :-20°C Freezer
solubility :DMSO (Slightly), Methanol (Slightly)
form :Yellow solid.
color :Light Yellow to Yellow
Safety Information
| Symbol(GHS): |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | |||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
Nintedanib esylate is a potent, oral triple angiokinase inhibitor developed by Boehringer Ingelheim that targets proangiogenic and pro-fibrotic pathways mediated by the vascular endothelial growth factor receptor, fibroblast growth factor receptor and plateletderived growth factor receptor families, as well as Src and Flt-3 kinases. It was approved for the treatment of idiopathic pulmonary fibrosis (IPF), a condition in which the lungs become progressively scarred over time, by the US FDA in October 2014 and by the EMA in January 2015. The FDA granted nintedanib esylate fast-track, priority review, orphan product, and breakthrough designations. The drug was also approved by the EMA in November 2014 for treatment of non-small cell lung cancer in combination with docetaxel after first-line chemotherapy.More related product prices
14192-26-8 Nintedanib 1,3-DiMethyl 2-[4-(Methoxycarbonyl)-2-nitrophenyl]propanedioate N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide Ruxolitinib phosphate Olaparib 918504-65-1 PLERIXAFOR Afatinib (BIBW 2992) Crizotinib Propylparaben Methylparaben N-Methyl-4-nitroaniline Sodium methylparaben Ethylparaben Butylparaben p-Hydroxybenzoic acid ethyl ester sodium saltRelated product price
- 14192-26-8
₹6800-18023.63 - PLERIXAFOR
₹4800-13800 - Crizotinib
₹13174.03






