PASIREOTIDE
|
- ₹0
- Product name: PASIREOTIDE
- CAS: 396091-73-9
- MF: C58H66N10O9
- MW: 1047.23
- EINECS:
- MDL Number:MFCD08067735
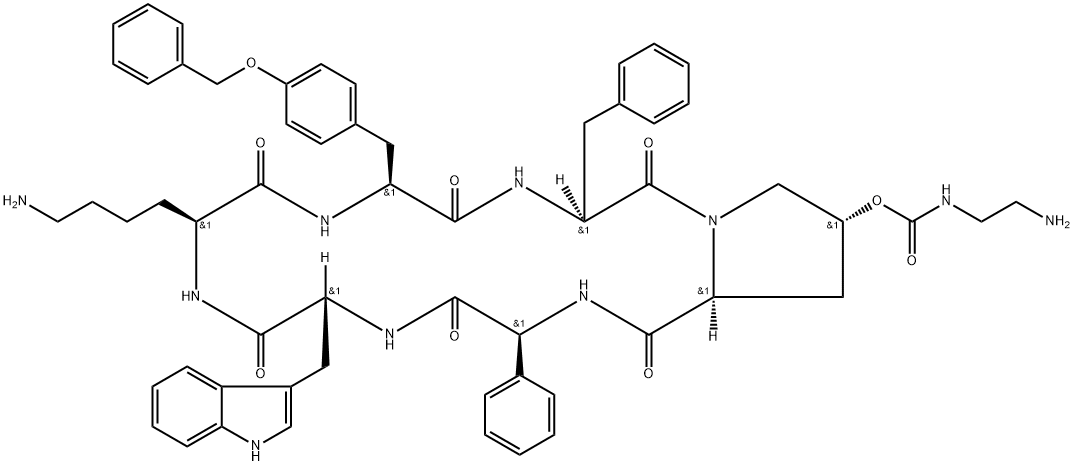
- Synonyms:PASIREOTIDE;Cyclo[(2S)-2-phenylglycyl-D-tryptophyl-L-lysyl-O-(phenylmethyl)-L-tyrosyl-L-phenylalanyl-(4R)-4-[[[(2-aminoethyl)amino]carbonyl]oxy]-L-prolyl];SOM 230;SOM 320;[(3S,6S,9S,12R,15S,18S,20R)-9-(4-aminobutyl)-3-benzyl-12-(1H-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-15-phenyl-6-[(4-phenylmethoxyphenyl)methyl]-1,4,7,10,13,16-hexazabicyclo[16.3.0]henicosan-20-yl] N-(2-aminoethyl)carbamate;SOM 230;SOM 320;CYCLO((4R)-4-(2-AMINOETHYLCARBAMOYLOXY)-L-PROLYL-L-PHENYLGLYCYL-D-TRYPTOPHYL-L-LYSYL-4-O-BENZYL-L-TYROSYL-L-PHENYLALANYL-);Pasireotide Acetate(net)
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Boiling point :1351.4±65.0 °C(Predicted)
Density :1.36±0.1 g/cm3(Predicted)
storage temp. :Store at -20°C
solubility :Soluble in DMSO
form :Powder
pka :11.86±0.46(Predicted)
Sequence :cyclo[Tyr(Bzl)-Phe-Hyp(Bom)-Phg-D-Trp-Lys]
CAS DataBase Reference :396091-73-9
Density :1.36±0.1 g/cm3(Predicted)
storage temp. :Store at -20°C
solubility :Soluble in DMSO
form :Powder
pka :11.86±0.46(Predicted)
Sequence :cyclo[Tyr(Bzl)-Phe-Hyp(Bom)-Phg-D-Trp-Lys]
CAS DataBase Reference :396091-73-9
Safety Information
| Symbol(GHS): |
  
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
In April 2012, the European Commission approved pasireotide for the treatment of Cushing’s Disease (CD) in adult patients who have not responded to surgical interventionor forwhomsurgery is not anoption.Pasireotide was approved for the same indication by the US FDA in December of 2012. Pasireotide (also known as SOM230) is a cyclohexapeptide that acts as a somatostatin analogue to inhibit the release of ACTH. Somatostatins are cyclic peptides of 14 and 28 amino acids that play animportant role inregulating endocrineandexocrine release inmany tissues through an inhibitory mechanism. There are five known subtypes of somatostatin receptors (SSTRs). Natural somatostatins bind with high affinity to all five subtypes, however, their therapeutic use is limited by rapid degradation in plasma. Pasireotide arose fromefforts to identify a somatostatinmimetic with long-lasting inhibitory effects. Starting with a 14-amino acid somatostatin peptide, a systematic alanine scan revealed residues that were essential for receptor sub-type binding, including key b-turn regions and adjacent residues. Placing the key structural elements as unnatural amino acids in a cyclohexapeptide backbone gave pasireotide.More related product prices
Etelcalcetide VAPREOTIDE TRH Sincalide Entacapone Liraglutide Capecitabine Somatostatin Oxytocin Octreotide acetate H-GLY-PRO-HYP-OHRelated product price
- TRH
₹14061.68-174747.98 - Sincalide
₹22093.83-231362.73 - Entacapone
₹7707.4-25958.35
Suppliers and manufacturers
CLEARSYNTH LABS LTD.
Pharma Affiliates
Pharmaffiliates Analytics and Synthetics P. Ltd
Alpha Biopharmaceuticals Co., Ltd
Shenzhen Nexconn Pharmatechs Ltd
BOC Sciences
Shanghai Longyu Biotechnology Co., Ltd.






