Raltegravir
|
- ₹0
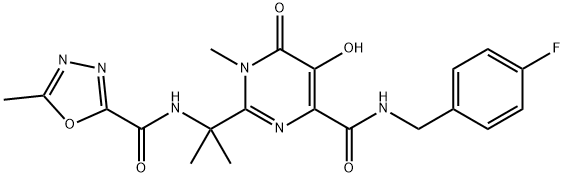
- Product name: Raltegravir
- CAS: 518048-05-0
- MF: C20H21FN6O5
- MW: 444.42
- EINECS:610-733-3
- MDL Number:MFCD10698872
- Synonyms:N-((4-Fluorophenyl)methyl)-1,6-dihydro-5-hydroxy-1-methyl-2-(1-methyl-1-(((5-methyl-1,3,4-oxadiazol-2;Raltegravir N-(2-(4-(4-Fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide;Raltegravir (free base) API;Raltegravir, >=99%;N-((4-Fluorophenyl)methyl)-1,6-dihydro-5-hydroxy-1-methyl-2-(1-methyl-1-(((5-methyl-1,3,4-oxadiazol-2-yl)carbonyl)amino)ethyl)-6-oxo-4-pyrimidinecarboxamide;N-(2-(4-(4-Fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide;Raltegravir;N-(2-(4-(4-Fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)p
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :216 °C(Solv: isopropanol (67-63-0))
Density :1.46±0.1 g/cm3(Predicted)
storage temp. :-20°C Freezer
solubility :DMSO (Slightly), Methanol (Very Slightly)
pka :4.50±1.00(Predicted)
form :Solid
color :White to Light Beige
CAS DataBase Reference :518048-05-0(CAS DataBase Reference)
Density :1.46±0.1 g/cm3(Predicted)
storage temp. :-20°C Freezer
solubility :DMSO (Slightly), Methanol (Very Slightly)
pka :4.50±1.00(Predicted)
form :Solid
color :White to Light Beige
CAS DataBase Reference :518048-05-0(CAS DataBase Reference)
Safety Information
| Symbol(GHS): |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
Joining maraviroc as a unique approach to battling HIV-1, raltegravir, an inhibitor of HIV-1 integrase, represents the first in its class to be developed and launched as a combination treatment with other antiretroviral agents (NRTIs, NNRTIs, and PIs). HIV-1 integrase is essential for replication of the virus as a virally encoded enzyme that integrates the viral DNA into the genome of the host cell. Inhibition of HIV-1 integrase prevents the two-step process of endonucleolytic removal of the terminal dinucleotide from each 3′end of the viral DNA followed by the covalent integration of the viral DNA, at these modified 3′ends, into the host DNA, thereby representing a viable intervention in the viral life cycle. In vitro, raltegravir inhibited the strand transfer activity of HIV-1 integrase with an IC50 of 2–7nM with > 1,000-fold selectivity over other phosphoryltransferases. In addition, its in vitro IC95 for HIV-1 in 10% fetal bovine serum and 50% human serum was 19 and 33 nM, respectively. Raltegravir was well tolerated with no dose-related toxicities and a safety profile comparable to placebo. The most common clinical adverse events were diarrhea, nausea, vomiting, fatigue, headache, flushing, pruritus, and injection-site reactions.Related product price
- Methylparaben
₹1386-57231.78 - 5380-42-7
₹3300-8800 - Bensulfuron methyl
₹10695.1






