SULCONAZOLE
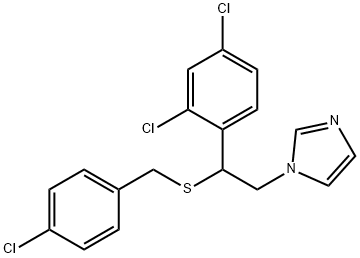
- CAS No.
- 61318-90-9
- Chemical Name:
- SULCONAZOLE
- Synonyms
- Sulconazol;SULCONAZOLE;SULCONAZOLE USP/EP/BP;Sulconazle Impurity 3;1-(2-((4-Chlorobenzyl);-2-(2,4-dichlorophenyl);Uniconazole sulfur nitrate;Quizalofop Impurity 8(Propaquizafop);Sulconazole (base and/or unspecified salts);1-(2-((4-Chlorobenzyl)thio)-2-(2,4-dichlorophenyl)ethyl)-1H-iMidazole
- CBNumber:
- CB1102530
- Molecular Formula:
- C18H15Cl3N2S
- Molecular Weight:
- 397.75
- MOL File:
- 61318-90-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2022/12/21 16:56:50
| Boiling point | 558.2±50.0 °C(Predicted) |
|---|---|
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| pka | 6.55±0.12(Predicted) |
| CAS DataBase Reference | 61318-90-9 |
SULCONAZOLE Chemical Properties,Uses,Production
Uses
Sulconazole (Exelderm) is a synthetic, sulfur-substituted imidazole that is structurally related to the other imidazole antifungals (ketoconazole, econazole, and miconazole). It is postulated to inhibit 14-α-demethylase for decreased ergosterol synthesis, increased cell membrane permeability, and resultant cell death. It is active against most dermatophytes, yeast, and P. orbiculare. It also displays activity against some aerobic and anaerobic gram-positive bacteria. Sulconazole is a mild inducer of the cytochrome P-450 microsomal enzymes CYP1A1 and CYP2B1 and could theoretically induce the metabolism of other drugs.
Indications
Sulconazole (Exelderm) is a synthetic, sulfur-substituted imidazole that is structurally related to the other imidazole antifungals (ketoconazole, econazole, and miconazole). It is postulated to inhibit 14-α-demethylase for decreased ergosterol synthesis, increased cell membrane permeability, and resultant cell death. It is active against most dermatophytes, yeast, and P. orbiculare. It also displays activity against some aerobic and anaerobic gram-positive bacteria. Sulconazole is a mild inducer of the cytochrome P-450 microsomal enzymes CYP1A1 and CYP2B1 and could theoretically induce the metabolism of other drugs.
Definition
ChEBI: A member of the class of imidazoles that is 1-ethyl-1H-imidazole in which one of the hydrogens of the methyl group is replaced by a (4-chlorobenzyl)sulfanediyl group while a second is replaced by a 2,4-dichlorophenyl group.
Indications
Topical treatment of mycoses of the skin induced or sustained by fungi such as dermatophytes and yeasts.
Side effects
Local irritations and allergic reactions may occur in rare cases and are mainly due to the galenic formulation.
Solubility in organics
1 part in 3,333 (water), 1 part in 100 (ethanol), 1 part in 130 (acetone), 1 part in 333 (chloroform), 1 part in 2000 (dioxan), 1 part in 71 (methanol), 1 part in 286(chloromethane), 1 part in 10 (pyridine), 1 part in 2000 (toluene).
SULCONAZOLE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
SULCONAZOLE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Gufic Group | +91-2267261000 +91-2267261000 | Maharashtra, India | 22 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Angle Biopharma | 08046067501 | Ahmedabad, India | 142 | 58 | Inquiry |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | China | 2141 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 24607 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
Related articles
- Pharmacodynamics of Sulconazole
- Sulconazole has a broad spectrum of antifungal activity in vitro and has been shown to be an effective topical antifungal agen....
- Mar 30,2022





