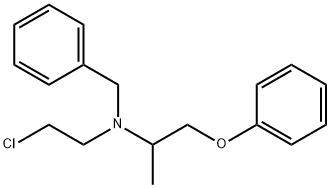
phenoxybenzamine
- CAS No.
- 59-96-1
- Chemical Name:
- phenoxybenzamine
- Synonyms
- Phenoxy;Dibenyline;Phenoxybenzamin;phenoxybenzamine USP/EP/BP;N-Benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-aMine;N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl)benzylamine;BENZYLAMINE,N-(2-CHLOROETHYL)-N-(1-METHYL-2-PHENOXYETHYL)-;Benzenemethanamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-
- CBNumber:
- CB1933947
- Molecular Formula:
- C18H22ClNO
- Molecular Weight:
- 303.83
- MOL File:
- 59-96-1.mol
- Modify Date:
- 2023/5/4 17:34:40
| Melting point | 38-40° |
|---|---|
| Boiling point | 381.5±27.0 °C(Predicted) |
| Density | 1.0513 (rough estimate) |
| refractive index | 1.5600 (estimate) |
| pka | 6.58±0.50(Predicted) |
| color | Crystals from pet ether |
| CAS DataBase Reference | 59-96-1 |
| EPA Substance Registry System | N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl)benzenemethanamine (59-96-1) |
phenoxybenzamine Chemical Properties,Uses,Production
Uses
Antihypertensive.
World Health Organization (WHO)
Phenoxybenzamine, a long-acting alpha-adrenoreceptor antagonist, was introduced in 1953 and has been used in a variety of peripheral vascular disorders. In 1982 it was shown to have mutagenic activity and in 1985 it was found to be carcinogenic in the rat. Its approved use was subsequently restricted by several regulatory authorities and phenoxybenzamine is currently used to manage hypertensive episodes associated with phaeochromocytoma, as an adjunct to the short-term management of urinary retention due to neurogenic bladder, in the short-term treatment of benign prostatic hypertrophy in patients awaiting surgery, and in inoperable benign prostatic hypertrophy.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intravenous and intracerebral routes. Moderately toxic by ingestion. Human reproductive effects by ingestion: spermatogenesis. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of Cland NOx.
Purification Methods
The free base is crystallised from pet ether, and the HCl is crystallised from EtOH/diethyl ether. [Beilstein 12 IV 2204.]
phenoxybenzamine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Mylan Laboratories Ltd | +91-4023550543 +91-4030866666 | Telangana, India | 150 | 58 | Inquiry |
| Apicore Pharmaceuticals Pvt Ltd | +91-2662267177 +91-2662267166 | Gujarat, India | 181 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Laffans Petrochemicals Limited | 09820085810 | Mumbai, India | 14 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | China | 9624 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49999 | 58 | Inquiry |
| Finetech Industry Limited | 027-87465837 19945049750 | China | 9618 | 58 | Inquiry |





