ZOTAROLIMUS
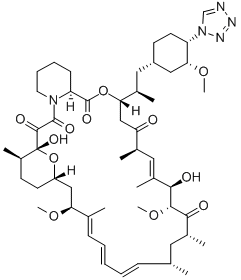
- CAS No.
- 221877-54-9
- Chemical Name:
- ZOTAROLIMUS
- Synonyms
- ABT 578;CS-1162;A 179578;Resolute;ZOTAROLIMUS;ZotaroliMus API;Unii-H4gxr80ize;Zotarolimus, >ZotaroliMus, >90%;ZotaroliMus(ABT-578)
- CBNumber:
- CB21011766
- Molecular Formula:
- C52H79N5O12
- Molecular Weight:
- 966.21
- MOL File:
- 221877-54-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/18 11:30:58
ZOTAROLIMUS Chemical Properties,Uses,Production
Description
Zotarolimus is a macrocyclic lactone immunosuppressant and a derivative of rapamycin . It binds to FKBP prolyl isomerase 1A (FKBP12; IC50 = 2.57 nM) and inhibits proliferation of human peripheral blood mononuclear cells (PBMCs), rat splenocytes, and human coronary artery smooth muscle cells (IC50s = 7, 1,337, and 0.8 nM, respectively). Zotarolimus has immunosuppressive activity in a one-way mixed lymphocyte reaction using human or rat lymphocytes (IC50s = 1.2 and 1,465 nM, respectively). It also reduces symptom severity in a rat model of experimental autoimmune encephalomyelitis (EAE; ED50 = 1.17 mg/kg per day) and delays cardiac allograft rejection in rats (ED50 = 3.71 mg/kg per day). Zotarolimus inhibits neointimal formation and reduces stenosis in pig coronary arteries when applied at 10 μg/mm to stainless steel balloon expandable stents with phosphorylcholine in a model of restenosis. Formulations containing zotarolimus have been used in drug-eluting stents in the prevention of restenosis following stent placement.
Chemical Properties
Pale Yellow Solid
Uses
Zotarolimus is a semi-synthetic macrocyclic lactone prepared from rapamycin by preparation of the 42-triflate ester, followed by displacement with tetrazole and purification of the two isomeric products. This structural change affords a less bioavailable product, a preferred profile for some applications. Like all tacrolimus analogues, zotarolimus binds to a receptor protein (FKBP12). The complex then binds to preventing it from interacting with target proteins. Zotarolimus is extensively cited in the literature with over 200 citations.
ZOTAROLIMUS Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9343 | 55 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 24099 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| LEAP CHEM CO., LTD. | +86-852-30606658 | China | 24738 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |





