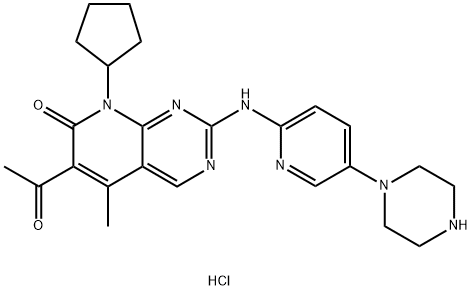
PD 0332991 HCl
- CAS No.
- 827022-32-2
- Chemical Name:
- PD 0332991 HCl
- Synonyms
- CS-365;PD 0332991 HCl;Palbociclib HCl;Palbociclib-025;Palbociclib-019-HCl;PD 0332991;PD-0332991;PD 0332991 hydrochloride;PD-0332991 (Palbociclib);PD 0332991 HCl USP/EP/BP;Palbociclib (hydrochloride)
- CBNumber:
- CB22593007
- Molecular Formula:
- C24H30ClN7O2
- Molecular Weight:
- 484
- MOL File:
- 827022-32-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:03
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
| HS Code | 29399990 |
PD 0332991 HCl price More Price(1)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 5.30487 | StemSelect PD 0332991 - CAS 827022-32-2 - Calbiochem A cell-permeable, brain permeant, potent, reversible, ATP competitive inhibitor of Cdk4 and Cdk6 (IC?? = 11, 9, and 15 nM for Cdk4/D1, Cdk4/D3 and Cdk | 827022-32-2 | 5MG | ₹12940 | 2022-06-14 | Buy |
| Product number | Packaging | Price | Buy |
|---|---|---|---|
| 5.30487 | 5MG | ₹12940 | Buy |
PD 0332991 HCl Chemical Properties,Uses,Production
Description
PD 0332991 is an orally active, selective inhibitor of the cyclin D kinases Cdk4 (IC50 = 11 nM) and Cdk6 (IC50= 16 nM) with no activity against a panel of 36 additional protein kinases. It has been reported to have antiproliferative activity against retinoblastoma-positive tumor cells, blocking retinoblastoma phosphorylation and inducing G1 arrest at nanomolar concentrations. PD 0332991 can inhibit the growth of certain ER-positive or HER2-amplified breast cancer cells (IC50s as low as 4 nM) and demonstrates synergy with tamoxifen and trastuzumab, respectively. PD 0332991 inhibition of Cdk4 activity has been used to demonstrate a role for insulin-activated cyclinD1-Cdk4 signaling in the control of glucose metabolism that is independent of cell cycle progression.
General Description
A cell-permeable, orally available and brain permeant, non-toxic pyridopyrimidinone compound that acts as a potent, selective, reversible, ATP competitive inhibitor of Cdk4 and Cdk6 (IC50 = 11, 9, and 15 nM for Cdk4/D1, Cdk4/D3 and Cdk6/D2, respectively). Hence, it reduces retinoblastoma protein phosphorylation at Ser780/Ser795 (IC50 = 66 nM in MDA-435 cells) and arrests cell cycle at G1 phase. Acts as a cytostatic agent, but does induce apoptotic cell death when used alone. However, it potentiates the cytotoxicity of dexamethasone (>Cat. No. 265005), bortezomib (>Cat. No. 504314), and tamoxifen (Cat. No. 579000) in estrogen receptor (ER)-positive cell lines. Exhibits only a trivial inhibitory activity towards Cdk2/E2, Cdk2/A, Cdk1/B and Cdk5/p25 in a 36-kinase panel (IC50 >10 μM). Improves endoderm differentiation of late G1-human embryonic stem cells expressing Smad2 or Smad3 (~ 750 nM) and further enhances endoderm differentiation into hepatic and pancreatic progenitor cells. Shown to regress the growth of human breast tumor xenografts in murine models (~150 mg/kg, p.o., daily).
Biochem/physiol Actions
Cell permeable: yes
PD 0332991 HCl Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20308 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9337 | 55 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29898 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
827022-32-2(PD 0332991 HCl)Related Search:
1of4
chevron_right




