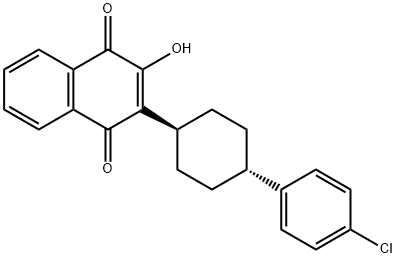
Atovaquone
- CAS No.
- 95233-18-4
- Chemical Name:
- Atovaquone
- Synonyms
- trans-2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione;2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthalenedione;Acuvel;BW-566C; 566C80;Wellvone;Atovatone;ATOVAQUONE;BW-566C-80;Atavaquone
- CBNumber:
- CB4383893
- Molecular Formula:
- C22H19ClO3
- Molecular Weight:
- 366.84
- MOL File:
- 95233-18-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/11 13:43:58
| Melting point | 216-2190C |
|---|---|
| Boiling point | 535.0±50.0 °C(Predicted) |
| Density | 1.349±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >10mg/mL |
| pka | 5.01±0.10(Predicted) |
| form | powder |
| color | yellow |
| λmax | 494nm(aq. Buffer)(lit.) |
| Merck | 14,866 |
| InChIKey | KUCQYCKVKVOKAY-CTYIDZIISA-N |
| CAS DataBase Reference | 95233-18-4(CAS DataBase Reference) |
Atovaquone price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | A7986 | Atovaquone ≥98% (HPLC) | 95233-18-4 | 10MG | ₹8075.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1591 | Atovaquone Pharmaceutical Secondary Standard; Certified Reference Material | 95233-18-4 | 500MG | ₹9103.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A7986 | Atovaquone ≥98% (HPLC) | 95233-18-4 | 50MG | ₹31717.25 | 2022-06-14 | Buy |
| TCI Chemicals (India) | A2545 | Atovaquone | 95233-18-4 | 200MG | ₹4100 | 2022-05-26 | Buy |
| TCI Chemicals (India) | A2545 | Atovaquone | 95233-18-4 | 1G | ₹8600 | 2022-05-26 | Buy |
Atovaquone Chemical Properties,Uses,Production
Description
Atovaquone is an orally active antiprotozoal agent indicated for patients with mild to moderate AIDS-associated Pneumocystis carinii pneumonia who are intolerant to the fist-line therapy of trimethoprim-sulfamethoxazole. It is also under investigation as a treatment for malaria and AIDS-associated toxoplasmosis.
Chemical Properties
Yellow to Orange Crystalline Solid
Uses
Atovaquone inhibits the cytochrome bc(1) complex via interactions with the Rieske iron-sulfur protein and cytochrome b in the ubiquinol oxidation pocket. In addition to its use as a treatment for toxoplasmosis, atovaquone has antimalarial properties and prevents pneumocystis pneumonia post-renal transplant.
Definition
ChEBI: A naphthoquinone compound having a 4-(4-chlorophenyl)cyclohexyl group at the 2-position and a hydroxy substituent at the 3-position.
Antimicrobial activity
It is active against erythrocytic, liver and sexual stages of malaria parasites. It shows synergy with proguanil and tetracyclines in vitro. It is also active against Babesia spp. and both tachyzoites and cysts of Tox. gondii. Pn. jirovecii is sensitive in vitro at 0.1–3.0 mg/L and high doses are effective in the rat.
Acquired resistance
Point mutations on parasite cytochrome b, in particular at codon 268, cause resistance and readily occur when the drug is used alone. The rapid selection of resistance led to the development of the synergistic combination with proguanil. Failure of Pn. jirovecii prophylaxis has also been associated with cytochrome b mutations.
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Atovaquone belongs to the class of naphthoquinones with a broad-spectrum activity against parasitic infections including malaria, toxoplasmosis and Pneumocystis pneumonia. It is a potent antiprotozoal compound which aids in collapsing the mitochondrial membrane potential in a malaria parasite.
Pharmaceutical Applications
A hydroxynaphthoquinone. Available as the trans isomer (which is more active than the cis form) for oral use. It is insoluble in water.
Mechanism of action
Atovaquone is thought to produce its antiparasitic action by virtue of its ability to inhibit the mitochondrial respiratory chain. More specifically, atovaquone is a ubiquinone reductase inhibitor, inhibiting at the cytochrome bc1 complex. This action leads to a collapse of the mitochondrial membrane potential. The compound shows stereospecific inhibition, with the trans isomer being more active than the cisisomer.
Pharmacokinetics
Oral absorption: Poor
Cmax 750 mg oral: 27 mg/L (steady state)
Plasma half-life: 70 h
Plasma protein binding: >99%
It is highly lipophilic and is poorly absorbed from the gastrointestinal
tract following oral administration. Bioavailability is
improved when administered with meals, particularly those
with a high fat content. Steady-state plasma concentrations
are up to 50% lower in AIDS patients than in asymptomatic
HIV-positive cases and the elimination half-life is lower (55 h)
in patients with AIDS. The concentration in CSF is <1% of
the plasma level. Unlike some other naphthoquinones it is not
metabolized by human liver microsomes. Combinations with
co-trimoxazole (in HIV patients) and with proguanil plus
artesunate in healthy adults did not produce any changes in
atovaquone pharmacokinetics.
Clinical Use
Pn. jirovecii pneumonia; alternative therapy for mild to moderate illness
(prophylaxis and treatment)
Prophylaxis and treatment of malaria in combination with proguanil
It has also been used in cerebral toxoplasmosis in AIDS
patients and in a few cases of human babesiosis.
Side effects
Most clinical trials of atovaquone alone have involved patients with AIDS in whom adverse effects are often difficult to detect; however, more than 20% reported fever, nausea, diarrhea and rashes. There were limited changes in hepatocellular function. In malaria, in combination with proguanil, there are few reported side effects.
Metabolism
Atovaquone is poorly absorbed from the GI tract because of its poor water solubility and high fat solubility, but the absorption can be significantly increased if taken with a fat-rich meal. The drug is highly bound to plasma protein (94%) and does not enter the CNS in significant quantities. It is not significantly metabolized in humans and is exclusively eliminated in feces via the bile.
Atovaquone Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| NGL Fine Chem limited | +91-8866366396 +91-8866366396 | Mumbai, India | 25 | 58 | Inquiry |
| USV Pvt Ltd | +91-2267861111 +91-2225564048 | Maharashtra, India | 36 | 58 | Inquiry |
| Glenmark Pharmaceuticals Limited | +912240189999 | Maharashtra, India | 93 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Nuray Chemicals Pvt Ltd | +91-4066616700 +91-8220444745 | Tamil Nadu, India | 49 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Aruvi Labs | +91-8056181939 +91-8056181939 | Tamil Nadu, India | 149 | 58 | Inquiry |
| Prachin Chemicals Saykha Private Limited | +91-9537763493 +91-9904988839 | Gujarat, India | 16 | 58 | Inquiry |
| APITORIA PHARMA PRIVATE LIMITED | +91 40 6672 5000 | Chongqing, India | 159 | 58 | Inquiry |
| TIANISH LABORATORIES PRIVATE LIMITED | 917995005494 | Chongqing, India | 98 | 58 | Inquiry |






