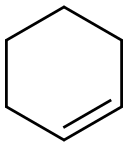
Cyclohexene
- CAS No.
- 110-83-8
- Chemical Name:
- Cyclohexene
- Synonyms
- HX;Cyclohexen;1-Cyclohexene;Hexanaphthylene;cykloheksen(polish);Cyclohexene, pure, 99%;ohexene;CYCLOHEXENE;Cykloheksen;JACS-110-83-8
- CBNumber:
- CB2852823
- Molecular Formula:
- C6H10
- Molecular Weight:
- 82.14
- MOL File:
- 110-83-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:26
| Melting point | ?104 °C (lit.) |
|---|---|
| Boiling point | 83 °C (lit.) |
| Density | 0.811 g/mL at 25 °C (lit.) |
| vapor density | 2.8 (vs air) |
| vapor pressure | 160 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 10 °F |
| storage temp. | Store below +30°C. |
| solubility | water: insoluble |
| form | Liquid |
| Specific Gravity | 0.811 |
| color | Clear colorless |
| PH | 7-8 (0.2g/l, H2O, 20℃) |
| explosive limit | 1.2-7.7%(V) |
| Water Solubility | insoluble |
| Merck | 14,2727 |
| BRN | 906737 |
| Henry's Law Constant | 3.85 x 10-2 atm?m3/mol at 25 °C (Nielsen et al., 1994) |
| Exposure limits | TLV-TWA 300 ppm (~1015 mg/m3) (ACGIH, OSHA, and NIOSH); IDLH 10,000 ppm (NIOSH). |
| Dielectric constant | 18.3(20℃) |
| Stability | Stable in the absence of air - may form peroxides in storage. Incompatible with oxidizing agents. Highly flammable. |
| InChIKey | HGCIXCUEYOPUTN-UHFFFAOYSA-N |
| LogP | 2.99 at 25℃ |
| CAS DataBase Reference | 110-83-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexene(110-83-8) |
| EPA Substance Registry System | Cyclohexene (110-83-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302-H304-H411 | |||||||||
| Precautionary statements | P210-P233-P240-P273-P301+P310-P331 | |||||||||
| Hazard Codes | F,Xn | |||||||||
| Risk Statements | 11-21/22-65 | |||||||||
| Safety Statements | 16-29-33-36/37-62 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 300 ppm (1015 mg/m3) | |||||||||
| RIDADR | UN 2256 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GW2500000 | |||||||||
| Autoignition Temperature | 590 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29021990 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1300 mg/kg | |||||||||
| IDLA | 2,000 ppm | |||||||||
| NFPA 704 |
|
Cyclohexene price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.02824 | Cyclohexene (stabilized) for synthesis | 110-83-8 | 1L | ₹2760 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.02824 | Cyclohexene (stabilized) for synthesis | 110-83-8 | 100ML | ₹4400.01 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.02824 | Cyclohexene (stabilized) for synthesis | 110-83-8 | 2.5L | ₹6160 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.02824 | Cyclohexene (stabilized) for synthesis | 110-83-8 | 500ML | ₹7240.01 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 44028 | Cyclohexene analytical standard | 110-83-8 | 5ML | ₹4264.13 | 2022-06-14 | Buy |
Cyclohexene Chemical Properties,Uses,Production
Description
Cyclohexene is a hydrocarbon, mostly obtained from the hydrogenation of benzene.
Chemical Properties
Cyclohexene is a colorless liquid (cyclic alkene) with a sweetish odor.
Physical properties
Clear, colorless liquid with a sweet odor. Odor threshold concentration is 0.18 ppm (quoted, Amoore and Hautala, 1983).
Uses
It occurs in coal tar and is obtained by catalyticdehydration of cyclohexanol and dehydrogenationof cyclohexane. Cyclohexene isused in making adipic acid, maleic acid,and butadiene; in oil extraction; as a stabilizerfor high-octane gasoline; and in organicsynthesis.
Production Methods
Cyclohexene is prepared by dehydration of cyclohexanol by thermal reaction of an ethylene–propylene–butadiene mixture (1).
Definition
ChEBI: A cycloalkene that is cylohexane with a single double bond.
General Description
A colorless liquid. Insoluble in water and less dense than water. Flash point 20°F. Vapors heavier than air. Inhalation of high concentrations may have a narcotic effect. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Cyclohexene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154].
Health Hazard
There are very few toxicological data availableon cyclohexene. Inhalation producesirritation of the eyes and respiratory tract. Itis also an irritant to skin. Its acute toxicityis low; the toxic effects are similar to thoseof cyclohexane. Exposure to high concentrationsor ingestion may cause drowsiness.Single exposures to 15,000 ppm of cyclohexenecould be lethal to rats.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by inhalation and ingestion. A very dangerous fire hazard when exposed to flame; can react with oxidizers. Dangerous; keep away fromheat and open flame. To fight fire, use foam, CO2, dry chemical.
Potential Exposure
May be used as an intermediate in making other chemicals (e.g., adipic acid, maleic acid, hex- ahydro benzoic acid), oil extraction and as a catalyst solvent.
Carcinogenicity
Cyclohexene was not mutagenic in Salmonella typhimurium with or without metabolic activation.
Shipping
UN2256 Cyclohexene, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Free cyclohexene from peroxides by washing with successive portions of dilute acidified ferrous sulfate, or with NaHSO3 solution, then with distilled water, drying with CaCl2 or CaSO4, and distilling under N2. Alternative methods for removing peroxides include passage through a column of alumina, refluxing with sodium wire or cupric stearate (then distilling from sodium). The diene is removed by refluxing with maleic anhydride before distilling under vacuum. Treatment with 0.1moles of MeMgI in 40mL of diethyl ether removes traces of oxygenated impurities. Other purification procedures include washing with aqueous NaOH, drying and distilling under N2 through a spinning band column, redistilling from CaH2, storing under sodium wire, and passing through a column of alumina, under N2, immediately before use. Store it at <0o under argon. [Coleman & Johnstone Org Synth Coll Vol I 83 1955, Carson & Ipatieff Org Synth Coll Vol II 152 1943, Woon et al. J Am Chem Soc 108 7990 1986, Wong et al. J Am Chem Soc 109 3428 1987.] [Beilstein 5 IV 218.]
Incompatibilities
Vapor may form explosive mixture with air. The substance can form explosive peroxides. The sub- stance may polymerize under certain conditions. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoa- cids, epoxides.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Cyclohexene Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Sri Neelima Laboratories | +91-7981733602 +91-9849736989 | Telangana, India | 205 | 58 | Inquiry |
| Vyshno Bio Sciences | 91-7095439993 | Hyderabad, India | 239 | 58 | Inquiry |
| Loba Chemie Pvt Ltd | +91-22-6663 6663 | Mumbai, India | 498 | 58 | Inquiry |
| Reax Chemicals | 08048372701Ext 416 | Hyderabad, India | 4223 | 58 | Inquiry |
| Meru Chem Pvt. Ltd. | 91-22-8037401801 | Maharashtra, India | 175 | 58 | Inquiry |
| Anand Agencies | 91-20-24454597 | Maharashtra, India | 2337 | 58 | Inquiry |
| Chika Pvt., Ltd. | 91-22-22821382 | Maharashtra, India | 9 | 58 | Inquiry |
| Sisco Resech Laboratories Private Limited | +91-22-4268 5800 | Mumbai, India | 655 | 58 | Inquiry |
| Golden Streak Laboratories Limited | +91 (40) 2726-0320 | New Delhi, India | 15 | 50 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Sri Neelima Laboratories | 58 |
| Vyshno Bio Sciences | 58 |
| Loba Chemie Pvt Ltd | 58 |
| Reax Chemicals | 58 |
| Meru Chem Pvt. Ltd. | 58 |
| Anand Agencies | 58 |
| Chika Pvt., Ltd. | 58 |
| Sisco Resech Laboratories Private Limited | 58 |
| Golden Streak Laboratories Limited | 50 |
| A.J Chemicals | 58 |
Related articles
- What is Cyclohexene?
- Cyclohexene with an active double bond is a cheap, abundant, and easily accessible raw material in industry, and can be synthe....
- Feb 26,2020
110-83-8(Cyclohexene)Related Search:
1of4
chevron_right




