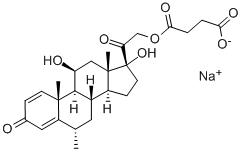
Methylprednisolone sodium succinate
- CAS No.
- 2375-03-3
- Chemical Name:
- Methylprednisolone sodium succinate
- Synonyms
- METHYLPREDNISOLONE SODIUM SUCCINATE;6α-methylprednisolone 21-hemisuccinate sodium salt;1,4-PREGNADIEN-6-ALPHA-METHYL-11-BETA, 17,21-TRIOL-3,20-DIONE 21-SUCCINATE, SODIUM SALT;6ALPHA-METHYL-11BETA,17ALPHA,21-TRIHYDROXY-1,4-PREGNADIENE-3,20-DIONE 21-HEMISUCCINATE SODIUM SALT;(6alpha,11beta)-21-(3-carboxy-1-oxopropoxy)-11,17-dihydroxy-6-methyl-pregna-1,4-diene-3,20-dione monosodium salt;u9088;Metypred;NSC 48989;succinate);solu-medrol
- CBNumber:
- CB5163715
- Molecular Formula:
- C26H33O8.Na
- Molecular Weight:
- 496.53
- MOL File:
- 2375-03-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | >186°C (dec.) |
|---|---|
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | lyophilized powder |
| color | White to Off-White |
| biological source | synthetic |
| Water Solubility | water: 50mg/mL, clear, colorless to faintly yellow |
| InChIKey | FQISKWAFAHGMGT-JXAFMBTDNA-M |
| SMILES | [C@]12(C[C@@H](C3=CC(=O)C=C[C@]3(C)[C@@]1([H])[C@H](C[C@]1(C)[C@@](CC[C@@]21[H])(O)C(=O)COC(=O)CCC([O-])=O)O)C)[H].[Na+] |&1:0,2,9,11,13,15,17,20,r| |
| CAS DataBase Reference | 2375-03-3(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H351-H360 | |||||||||
| Precautionary statements | P201-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 61-40 | |||||||||
| Safety Statements | 53-22-36/37/39-45 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TU4154060 | |||||||||
| Toxicity | LD50 oral in rat: > 5gm/kg | |||||||||
| NFPA 704 |
|
Methylprednisolone sodium succinate price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | M3781 | 6α-Methylprednisolone 21-hemisuccinate sodium salt lyophilized powder | 2375-03-3 | 25MG | ₹7826.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M3781 | 6α-Methylprednisolone 21-hemisuccinate sodium salt lyophilized powder | 2375-03-3 | 250MG | ₹39197.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M3781 | 6α-Methylprednisolone 21-hemisuccinate sodium salt lyophilized powder | 2375-03-3 | 1G | ₹105013.33 | 2022-06-14 | Buy |
Methylprednisolone sodium succinate Chemical Properties,Uses,Production
Description
Methylprednisolone Sodium Succinate is the sodium succinate salt of a synthetic glucocorticoid receptor agonist with immunosuppressive and anti-inflammatory effects. It is converted into active prednisolone in the body, which activates glucocorticoid receptor-mediated gene expression. This includes inducing the synthesis of the anti-inflammatory protein IkappaB-alpha and inhibiting the synthesis of nuclear factor kappa B (NF-kappaB). As a result, proinflammatory cytokine production, such as IL-1, IL-2, and IL-6, is down-regulated, and cytotoxic T-lymphocyte activation is inhibited. Therefore, an overall reduction in chronic inflammation and autoimmune reactions may be achieved.
Uses
6α-Methylprednisolone 21-Hemisuccinate is a glucocorticosteroid used in anti-inflammatory medication in the treatment of spinal cord injuries.
brand name
A-Methapred (Abbott); A-Methapred (Hospira); Solu-Medrol (Pharmacia & Upjohn).
Biochem/physiol Actions
Glucocorticoid with slightly longer half-life than that of prednisolone.
Side effects
Methylprednisolone Sodium Succinate is commonly given by injection and common side effects include: injection site pain/redness/swelling, nausea, vomiting, heartburn, headache, dizziness, difficulty sleeping, appetite changes, increased sweating, acne, increased blood sugar, sore throat, fever, chills, cough, white spots in the mouth. Serious side effects manifest as abnormal weight gain, menstrual disorders, bone/joint pain, easy bruising/bleeding, mental/mood changes, fast/slow/irregular heartbeat, stomach/intestinal bleeding symptoms, seizures, liver problems, and symptoms of severe allergic reactions. Contact your doctor if any of these serious adverse symptoms occur.
Methylprednisolone sodium succinate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Prachem Laboratories | +91-7948993109 +91-7383072020 | Gujarat, India | 44 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Horster Biotek Pvt Ltd | +91-7359657360 +91-9898127219 | Gujarat, India | 58 | 58 | Inquiry |
| Manus Aktteva | +91 (79) 6512-3395 | New Delhi, India | 581 | 34 | Inquiry |
| LUPIN LTD | +91 124 4885000 / +91 124 3325000 | New Delhi, India | 191 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Biovenice Criticure | 08048989255 | Haryana, India | 3 | 58 | Inquiry |
| Lakeview Medical Stores | 08048971790 | Gujarat, India | 2 | 58 | Inquiry |
| Himalaya Health Care | 08047636187 | Hyderabad, India | 1 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| Supplier | Advantage |
|---|---|
| Prachem Laboratories | 58 |
| Ralington Pharma | 58 |
| Horster Biotek Pvt Ltd | 58 |
| Manus Aktteva | 34 |
| LUPIN LTD | 58 |
| Pharmaffiliates Analytics and Synthetics P. Ltd | 58 |
| Biovenice Criticure | 58 |
| Lakeview Medical Stores | 58 |
| Himalaya Health Care | 58 |
| CHEMSWORTH | 30 |
2375-03-3(Methylprednisolone sodium succinate)Related Search:
1of4
chevron_right




