TROLEANDOMYCIN
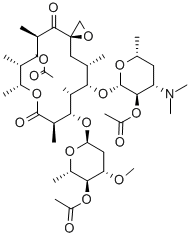
- CAS No.
- 2751-09-9
- Chemical Name:
- TROLEANDOMYCIN
- Synonyms
- tao;Taocin;Aovine;AI3-50166;evramicina;NSC-108166;cyclamycin;Matromycin T;Mathromycin T;TROLEANDOMYCIN
- CBNumber:
- CB5312403
- Molecular Formula:
- C41H67NO15
- Molecular Weight:
- 813.97
- MOL File:
- 2751-09-9.mol
- Modify Date:
- 2024/3/16 16:25:34
| Melting point | 170 °C |
|---|---|
| alpha | D25 -23° (methanol) |
| Boiling point | 757°C (rough estimate) |
| Density | 1.1651 (rough estimate) |
| refractive index | 1.6220 (estimate) |
| storage temp. | 0-6°C |
| solubility | Soluble in DMSO (up to 50 mg/ml) or in Ethanol (up to 25 mg/ml). |
| form | White solid. |
| pka | 6.6(at 25℃) |
| color | White |
| Water Solubility | 0.25g/L(28 ºC) |
| Merck | 13,9839 |
| Stability | Stable for 2 years as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H332-H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P-P261-P271-P304+P340-P312 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 2 |
| RTECS | RJ9900000 |
| F | 8 |
| Toxicity | LD50 oral in mouse: > 5gm/kg |
TROLEANDOMYCIN Chemical Properties,Uses,Production
Uses
Troleandomycin, is a macrolide antibiotic, sold in Italy and Turkey. It is a derivative of Oleandomycin (O517500), and acts as a CYP3A4 inhibitor, which may cause drug interactions .
Definition
ChEBI: A semi-synthetic macrolide antibiotic obtained by acetylation of the three free hydroxy groups of oleandomycin. Troleandomycin is only found in individuals that have taken the drug.
General Description
Triacetyloleandomycin was synthesized by Pfizer Research Laboratories in 1958. It shows a higher and longer-lasting serum level than oleandomycin when administered orally. Triacetyloleandomycin behaves as oleandomycin in vivo, following its hydrolysis by intestinal esterase. However, considerable amounts of the intermediates, the monoacetates and diacetates, are detected in the serum and urine. Prolonged use of triacetyloleandomycin causes damage to the liver as does erythromycin estolate.
Clinical Use
Oleandomycin contains three hydroxyl groups that aresubject to acylation, one in each of the sugars and one in theoleandolide. The triacetyl derivative retains the in vivo antibacterialactivity of the parent antibiotic but possesses superiorpharmacokinetic properties. It is hydrolyzed in vivo tooleandomycin. Troleandomycin achieves more rapid andhigher plasma concentrations following oral administrationthan oleandomycin phosphate, and it has the additionaladvantage of being practically tasteless. Troleandomycinoccurs as a white, crystalline solid that is nearly insoluble inwater. It is relatively stable in the solid state but undergoeschemical degradation in either aqueous acidic or alkalineconditions.
Because the antibacterial spectrum of activity of oleandomycinis considered inferior to that of erythromycin, thepharmacokinetics of troleandomycin have not been studiedextensively. Oral absorption is apparently good, and detectableblood levels of oleandomycin persist up to 12 hoursafter a 500-mg dose of troleandomycin. Approximately 20%is recovered in the urine, with most excreted in the feces, primarilyas a result of biliary excretion. There is some epigastricdistress following oral administration, with an incidencesimilar to that caused by erythromycin. Troleandomycin isthe most potent inhibitor of cytochrome P450 enzymes of thecommercially available macrolides. It may potentiate the hepatictoxicity of certain anti-inflammatory steroids and oralcontraceptive drugs as well as the toxic effects of theophylline,carbamazepine, and triazolam. Several allergic reactions,including cholestatic hepatitis, have also been reportedwith the use of troleandomycin.
Approved medical indications for troleandomycin arecurrently limited to the treatment of upper respiratory infectionscaused by such organisms as S. pyogenes and S. pneumoniae.It may be considered an alternative to oral forms oferythromycin. It is available in capsules and as a suspension.
TROLEANDOMYCIN Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 8444 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 52927 | 58 | Inquiry |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 | China | 465 | 58 | Inquiry |
| Artis Biotech Co. Ltd. | 19138486554 18582380095 | China | 2765 | 58 | Inquiry |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 15801484223; | China | 29778 | 58 | Inquiry |
| cjbscvictory | 13348960310 13348960310 | China | 10000 | 58 | Inquiry |





