oleandomycin
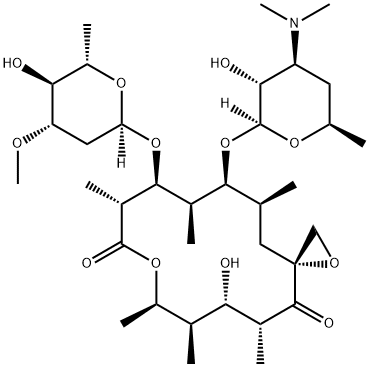
- CAS No.
- 3922-90-5
- Chemical Name:
- oleandomycin
- Synonyms
- C01946;PA 775;PA-105;Romicil;Amimycin;Landomycin;oleandomycin;OleandoMycin A;Antibiotic PA-105;AMiMycin, MatroMycin, PA 105
- CBNumber:
- CB5928730
- Molecular Formula:
- C35H61NO12
- Molecular Weight:
- 687.86
- MOL File:
- 3922-90-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/18 11:31:11
oleandomycin Chemical Properties,Uses,Production
Uses
Antibiotic substance produced by Streptomyces antibioticus no. ATCC 11891. Antibacterial.
Pharmaceutical Applications
A natural 14-membered-ring macrolide produced by Streptomyces antibioticus. It is stable in acid conditions. It is less active than erythromycin A in vitro, but four times more active than spiramycin . Several attempts have been made to improve its potency by chemical modification while retaining its relative acid stability.
It is incompletely absorbed, but an ester, triacetyloleandomycin, gives improved plasma levels. Following doses of 0.5 g, mean peak serum levels around 0.8 mg/L were reached by the base and 2 mg/L by the triacetyl ester. A single oral dose of 1 g of the ester produced a mean plasma oleandomycin concentration of 4 mg/L at 1 h after dosing, with an AUC of 14 mg.h/L. The apparent elimination half-life was 4.2 h. Significant quantities of mostly inactivated compound are eliminated in the bile. About 10% of the dose appears in the urine after administration of the base and about 20% after the ester.
Nausea, vomiting and diarrhea are common. Like erythromycin estolate, triacetyloleandomycin can cause liver damage. Abnormal liver function tests were found in about one-third of patients treated for 2 weeks. Hepatic dysfunction resolved when treatment was discontinued. The action of drugs eliminated via the cytochrome P450 system may be potentiated.
Its uses are similar to those of erythromycin. It is of restricted availability.
Safety Profile
A poison by intravenous route. Low toxicity by ingestion. When heated to decomposition it emits toxic vapors of NOx.
oleandomycin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | China | 10297 | 58 | Inquiry |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | China | 5738 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | China | 10978 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 52927 | 58 | Inquiry |
3922-90-5(oleandomycin)Related Search:
1of4
chevron_right




