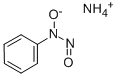
Cupferron
- CAS No.
- 135-20-6
- Chemical Name:
- Cupferron
- Synonyms
- NPHA;N-NITROSO-N-PHENYLHYDROXYLAMINE AMMONIUM SALT;Cupferon;Kupferron;CUPFERRON,REAGENT,ACS;N-Nitrosophenylhydroxylamine;N-NITROSO-N-PHENYLHYDROXYLAMINE;Kupferon;CUPFERRON;COPPERONE
- CBNumber:
- CB5679513
- Molecular Formula:
- C6H9N3O2
- Molecular Weight:
- 155.15
- MOL File:
- 135-20-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/8 18:06:34
| Melting point | 150-155 °C (dec.) (lit.) |
|---|---|
| Boiling point | 278.95°C (rough estimate) |
| Density | 1.3092 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | 2-8°C |
| solubility | well soluble in water and ethanol. |
| form | Powder |
| color | Needles from water |
| Water Solubility | <0.1 g/100 mL at 18.5 ºC |
| Merck | 14,2622 |
| BRN | 3919107 |
| Stability | Stable, but may be moisture sensitive. Incompatible with strong oxidizing agents, strong bases, strong acids. |
| InChIKey | GDEBSAWXIHEMNF-UHFFFAOYSA-O |
| CAS DataBase Reference | 135-20-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Cupferron(135-20-6) |
| IARC | 2B (Vol. 127) |
| EPA Substance Registry System | Cupferron (135-20-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H319-H335-H341-H350 | |||||||||
| Precautionary statements | P201-P202-P261-P264-P301+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25-36/37/38-40-45-43-23/24/25 | |||||||||
| Safety Statements | 26-36/37-45-53 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NC4725000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29299090 | |||||||||
| Toxicity | TDLo orl-rat: 123 g/kg/78W-C:CAR NCITR* NCI-CGTR-100,78 | |||||||||
| NFPA 704 |
|
Cupferron price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 675636 | Cupferron 97%, reagent grade | 135-20-6 | 100G | ₹19680 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 675636 | Cupferron 97%, reagent grade | 135-20-6 | 500G | ₹58470 | 2022-06-14 | Buy |
| TCI Chemicals (India) | C0646 | Cupferron | 135-20-6 | 25G | ₹2000 | 2022-05-26 | Buy |
| TCI Chemicals (India) | C0646 | Cupferron | 135-20-6 | 500G | ₹17300 | 2022-05-26 | Buy |
| ALFA India | ALF-A16551-14 | Cupferron, 97+% | 135-20-6 | 25g | ₹2177 | 2022-05-26 | Buy |
Cupferron Chemical Properties,Uses,Production
Chemical Properties
Cupferron is a creamy-white crystalline compound
Uses
The reagent used to be considered as specific for iron(II) and copper(II). However,
in the course of later analytical studies it turned out that cupferron forms
complexes with several other metals too, even in acidic medium. It is used today
primarily for the determination of copper(II), but it has proved suitable for the
determination of aluminium(III), bismuth(III), iron(III), mercury(II), thorium(IV),
tin(IV), titanium(III), vanadium(IV) and zirconium(IV) as well. Of these, the
zirconium(IV) complex is the most stable. Cupferron precipitates zirconium(IV)
quantitatively from aqueous media containing sulfuric acid.
The reagent gives a precipitate with most of the above metal ions even in strong
mineral acidic medium. Only the aluminium(III) complex does not precipitate in
the presence of mineral acids.
By means of cupferron many metal ions can be separated: thus, for instance,
iron(III) can be separated from aluminium(III) and manganese(II) and titanium
(IV), zirconium(IV) and hafnium(IV) from many other metal ions, etc. Though
the reagent makes possible several separations, the precipitates are not exactly of
stoichiometric composition (they almost always contain some excess of the ligand).
Hence instead of being directly weighed, the precipitates are usually ignited and
the metal oxide residues weighed.
General Description
Light yellow or cream-colored crystals or a brown crystalline solid. As a reagent in the separation of copper and iron.
Air & Water Reactions
Hygroscopic. Soluble in water.
Reactivity Profile
Cupferron may be sensitive to prolonged exposure to air. Incompatible with strong oxidizing agents, strong acids and strong bases. Forms unstable solutions with thorium, titanium and zirconium salts.
Fire Hazard
Flash point data for Cupferron are not available; however, Cupferron is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Poison by intravenous route. An eye irritant. Solutions with thorium salts are unstable explosives above 15°C. Solutions with titanium or zirconium salts are unstable explosives above 40℃. When heated to decomposition it emits very toxic NH3 and NOx. See also N-NITROSO COMPOUNDS and AMINES
Potential Exposure
Cupferron is used to separate tin from zinc, and copper and iron from other metals in the laboratory. Cupferron also finds application as a quantitative reagent for vanadates and titanium; and for the colorimetric determination of aluminum. The potential for exposure appears to be greatest for those engaged in analytical or research studies involving use of the chemical. Workers may also be exposed to the compound during manufacturing processes.
Carcinogenicity
Cupferron is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Recrystallise it twice from EtOH after treatment with Norite and finally once with EtOH. The crystals are washed with diethyl ether and air dried, then stored in the dark over solid ammonium carbonate. A standard solution (ca 0.05M prepared in air-free H2O) is prepared daily from this material for analytical work and is essentially 100% pure. [Olsen & Elving Anal Chem 26 1747 1954.] It can also be washed with Et2O, dried and stored as stated. In a sealed, dark container it can be stored for at least 12 months without deterioration. 260nm (CHCl3). max [Marvel Org Synth Coll Vol I 177 1941, Elving & Olson J Am Chem Soc 78 4206 1956, Beilstein 16 IV 891.] Possible CARCINOGEN.
Incompatibilities
Forms unstable and possibly explosive compounds with thorium salts; titanium, zirconium.
Cupferron Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Arnish Laborates Pvt Ltd | +91 7208475595 | Maharashtra, India | 187 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Anubhav Business Corporation | +91-2228014519 +91-2228014999 | Maharashtra, India | 119 | 58 | Inquiry |
| DeFINE CHEMICALS | +91-8010769889 +91-9320057099 | Maharashtra, India | 120 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| S. Nihar Exports | 91 22 208 5632 | New Delhi, India | 232 | 50 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1996 | 38 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1681 | 43 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Arnish Laborates Pvt Ltd | 58 |
| JSK Chemicals | 58 |
| Anubhav Business Corporation | 58 |
| DeFINE CHEMICALS | 58 |
| S D Fine Chem Limited | 58 |
| S. Nihar Exports | 50 |
| Scientific OEM | 38 |
| ALPHA CHEMIKA | 43 |
| A.J Chemicals | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
135-20-6(Cupferron)Related Search:
1of4
chevron_right




