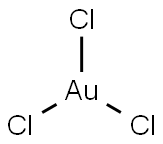
Gold(III) chloride
- CAS No.
- 13453-07-1
- Chemical Name:
- Gold(III) chloride
- Synonyms
- Gold trichloride;GoL;TETRACHLOROAURIC ACID;Trichlorogold;Gold(III) trichloride;d(III) chL;auricchloride;CHLORAUIC ACID;aurictrichloride;Gold(III) chloride
- CBNumber:
- CB5853063
- Molecular Formula:
- AuCl3
- Molecular Weight:
- 303.33
- MOL File:
- 13453-07-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/7/31 12:14:15
| Melting point | 254°C |
|---|---|
| Boiling point | 265 °C |
| Density | 3.9 g/mL at 25 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO (Sparingly), Methanol (Very Slightly) |
| form | Crystalline Powder, Crystals or Chunks |
| color | yellow |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4521 |
| Solubility Product Constant (Ksp) | pKsp: 24.5 |
| CAS DataBase Reference | 13453-07-1(CAS DataBase Reference) |
| EPA Substance Registry System | Gold chloride (AuCl3) (13453-07-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36/37/39-45-37/39-36 | |||||||||
| RIDADR | UN 3260 8/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MD5420000 | |||||||||
| F | 3-8-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28433000 | |||||||||
| NFPA 704 |
|
Gold(III) chloride price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 379948 | Gold(III) chloride ≥99.99% trace metals basis | 13453-07-1 | 250MG | ₹5916.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 379948 | Gold(III) chloride ≥99.99% trace metals basis | 13453-07-1 | 1G | ₹17704.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 334049 | Gold(III) chloride 99% | 13453-07-1 | 500MG | ₹8650 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 334049 | Gold(III) chloride 99% | 13453-07-1 | 5G | ₹43967.1 | 2022-06-14 | Buy |
| ALFA India | ALF-043360-06 | Gold(III) chloride, Premion™, 99.99% (metals basis), Au 64.4% min | 13453-07-1 | 5g | ₹51801 | 2022-05-26 | Buy |
Gold(III) chloride Chemical Properties,Uses,Production
Chemical Properties
orange-red to dark red crystals
Physical properties
Red monoclinic crystals; deliquesces; density 4.7 g/cm3; sublimes at 180°C (760 torr); highly soluble in water; soluble in alcohol and ether; slightly soluble in liquid ammonia.
Uses
Gold(III) chloride is used in colloidal gold solutions, in photography and as a print toning agent(gold toning), starting solution to form other gold compounds and a precursor for preparation of ultra pure gold metal. It is used in electroplating and electroless plating as an anode in an electric cell. Gold(III) chloride acts as a gold catalyst and cell body stains for bright field and dark field microscopy.
Definition
A compound prepared by dissolving gold in aqua regia. The bright yellow crystals (chloroauric acid) produced on evaporation are heated to form dark red crystals of gold(III) chloride. The chloride decomposes easily (at 175°C) to give gold(I) chloride and chlorine; at higher temperatures it decomposes to give gold and chlorine. Gold(III) chloride is used in photography. It exists as a dimer, Au2Cl6.
Preparation
Gold(III) chloride may be produced by the combination of metallic gold with chlorine gas at elevated temperatures:
2Au + 3Cl2 → 2AuCl3
It may be prepared in the laboratory by the reaction of iodine monochloride with metallic gold:
2Au + 6ICl → 2AuCl3 + 3I2
The compound should be stored tightly closed and protected from light.
Reactions
When heated at 254ºC, gold(III) chloride decomposes to gold(I) chloride and chlorine.
Passing hydrogen sulfide into an ether solution of the compound yields gold(III) sulfide, Au2S3.
A similar reaction occurs when alcoholic solutions of gold(III) chloride and hydrogen selenide are mixed, producing gold(III) selenide, Au2Se3, a black amorphous solid.
Gold(III) chloride may be reduced readily to metallic gold by common reducing agents. Thus, reduction with stannous chloride in dilute aqueous medium yields colloidal gold in which the atom carries a negative charge. “Cassius purple” is produced from the oxidation of tin to form H2Sn(OH)6, which protects colloidal gold from coagulation, imparting ruby red color to the solution.
Gold(III) chloride reacts with ammonia forming a gold(III)-nitrogen derivative, an explosive product, known as, “fulminate of gold”. Reaction with Grignard reagent, RMgX in ether yields dialkyl gold(III) chloride, R2AuCl3, which may be converted readily to other dialkyl gold(III) complexes by replacement of the chloride anion by a donor ligand.
General Description
The structure of gold chloride is monoclinic in nature. It sublimes at elevated temperatures.
Safety Profile
Experimental reproductive effects. Human mutation data reported. Reaction with ammonia or ammonium salts yields fulminating gold, a heat-, friction-, and impact-sensitive explosive similar to mercury and silver fulminates. See also GOLD COMPOUNDS and CHLORIDES. When heated to decomposition it emits toxic fumes of Cl-.
Gold(III) chloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| INDIAN PLATINUM PRIVATE LIMITED | +91-9322395199 +91-9322395199 | Mumbai, India | 54 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Akshar Noble Pvt Ltd | 08048250468Ext 541 | Gujarat, India | 22 | 58 | Inquiry |
| Prahas Healthcare LLP | 08048262123 | Vadodara, India | 10 | 58 | Inquiry |
| Souvenier Chemicals | 08048372762Ext 645 | Mumbai, India | 396 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| INDIAN PLATINUM PRIVATE LIMITED | 58 |
| Evans Fine Chem | 58 |
| Dhara Industries | 58 |
| A.J Chemicals | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Triveni chemicals | 58 |
| Akshar Noble Pvt Ltd | 58 |
| Prahas Healthcare LLP | 58 |
| Souvenier Chemicals | 58 |





