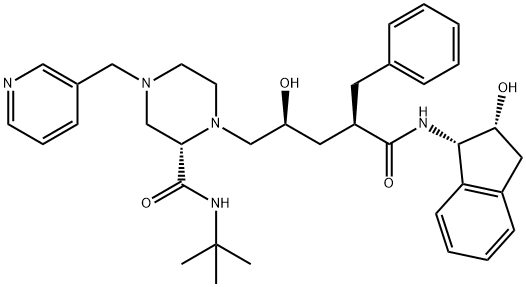
Indinavir
- CAS No.
- 150378-17-9
- Chemical Name:
- Indinavir
- Synonyms
- L-735524;INDINAVIR;Indinavir&Int.;MK-639:Cfixivan;Indinavir(MK-639;Indinavir USP/EP/BP;(1(1S,2R)5(S))-2,3,5-TRIDEOXY-N-(2,3-DIHYDRO-2HYDROXY-1H-INDEN-1-YI)-;[I(1S,2R),5(S)]-2,3,5-Trideoxy-N-(2,3.Dihydro-2-hydrox-Lh-inden-1-y1)-5-[2-[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide;(1(1S,2R),5(S))-2,3,5-Trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-(2-(((1,1-dimethylethyl)amino)carbonyl)-4-(3-pyridinylmethyl)-1-piperazinyl)-2-(phenylmethyl)-D-erythro-pentonamide
- CBNumber:
- CB6123497
- Molecular Formula:
- C36H47N5O4
- Molecular Weight:
- 613.79
- MOL File:
- 150378-17-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/21 10:59:17
| Melting point | 153-154°; mp 167.5-168° |
|---|---|
| alpha | D22 +24.1° (c = 0.0133 in chloroform) |
| Boiling point | 877.9±65.0 °C(Predicted) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | pKa 3.8/6.2(H2O,t undefined,Iundefined) (Uncertain) |
| Water Solubility | 70mg/L(temperature not stated) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Indinavir Chemical Properties,Uses,Production
Description
Crixivan was launched in Australia, Switzerland, the UK and the US as an orally-bioavailable HIV-1 protease inhibitor. The compound can be prepared by coupling an optically active piperazine derivative with an epoxide derivative (now commercially available). The synthesis of the proteins, reverse transcriptase, integrase, structural proteins and a protease, required by the virus to complete its lifecycle, can be interupted if the protease enzyme is not capable of cleaving a proform polypeptide chain into these components. lndinavir inhibits this process and is more potent than the first approved protease inhibitor saquinavir. This effect was noted by the increase in CD4+ cells and a decrease in HIV RNA levels. Since indinavir is metabolized by the CYP3A4 isozyme, care must be taken with patients with hepatic insufficiency and to sex-related differences in the level of this enzyme. Other than nephrolithiasis (5%), indinavir is relatively safe and well tolerated.
Indications
Indinavir (Crixivan) is a potent inhibitor of HIV reverse transcriptase. It produces the side effects common to all protease inhibitors and also may produce nephrolithiasis, urolithiasis, and possibly renal insufficiency or renal failure. This problem occurs more frequently in children (approximately 30%) than adults (approximately 10%) and can be minimized by drinking at least 1.5 L of water daily. Additional side effects include asymptomatic hyperbilirubinemia, alopecia, ingrown toenails, and paronychia. Hemolytic anemia rarely occurs. Rifampin should not be given with indinavir.
Acquired resistance
The major mutations in the protease enzyme associated with loss of the antiretroviral activity occur at positions 46, 82 and 84. Generally, the level of resistance rises with the number of point mutations.
General Description
When administered with a high-fat diet, indinavir(Crixivan) achieves a maximum serum concentration of77% of the administered dose. The drug is 60% bound inthe plasma. It is extensively metabolized by CYP3A4, andseven metabolites have been identified. Oral bioavailabilityis good, with a tmax of 0.8±0.3 hour. The half-life ofelimination is 1.8 hour, and the elimination products aredetectable in feces and urine. Indinavir also causes dyslipidemia.The available dosage forms are capsules of 200 mg,333 mg, and 400 mg.
Pharmaceutical Applications
A synthetic compound formulated as the sulfate for oral administration.
Pharmacokinetics
Oral absorption: c. 65%v
Cmax 800 mg thrice daily: c. 8.97 mg/L
Cmin 800 mg thrice daily: c. 0.15 mg/L
Plasma half-life: c. 2 h
Volume of distribution: c. 0.4–1.74 L/kg
Plasma protein binding: c. 60%
Absorption and distribution
It is rapidly absorbed and not significantly affected by intake with food. Distribution in the body has not been fully characterized. It penetrates well into the CNS. The semen:plasma ratio is 1.9. It is distributed into breast milk.
Metabolism and excretion
Seven major metabolites have been described, including a glucuronide conjugate and six oxidative metabolites. Around 83% of the dose is recovered in feces and 18% in urine, 10% as unchanged drug. The effect of renal impairment has not been studied. It should be used with caution in the presence of hepatic impairment, particularly if severe.
Clinical Use
Treatment of adult HIV infection (in combination with other antiretroviral drugs)
Side effects
The principal side effect is nephrolithiasis, including flank pain
with or without hematuria. There is good evidence that indinavir
directly causes nephrolithiasis as a result of crystallization
in the urinary tract. Indirect hyperbilirubinemia occurs in
about 10% of patients associated with inhibition of bilirubinconjugating
activity occurring as a result of competitive inhibition
of uridine diphosphate (UDP)-glucuronosyltransferase.
Ritonavir-boosted indinavir is associated with a dyslipidemia
profile characteristic of those treated with other
protease
inhibitors boosted with a 200 mg dose of ritonavir
per day. Insulin resistance and hyperglycemia have also been
associated with ritonavir-boosted indinavir.
Metabolism
The usual oral dose for indinavir alone or in combination with other antiviral agents is one 800 mg capsule every 8 hours. The drug is well absorbed if given on an empty stomach or 1 hour before or 2 hours after a light meal with water. The dose is reduced to 600 mg every 8 hours if given concurrently with ketoconazole. Indinavir activity is increased when combined with RT inhibitors.
Indinavir Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6211 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Greenwell Pharma | 08046068140 | Mumbai, India | 4 | 58 | Inquiry |
| Chandra Prabhu Pharma Services | 08048372381Ext 506 | Maharashtra, India | 19 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29898 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 24722 | 58 | Inquiry |
Related articles
- Side effects of Indinavir
- Indinavir is formulated as a sulfate salt and is available from the manufacturer for oral administration in strengths of 100, ....
- Apr 1,2022
150378-17-9(Indinavir)Related Search:
1of4
chevron_right




