Indiplon
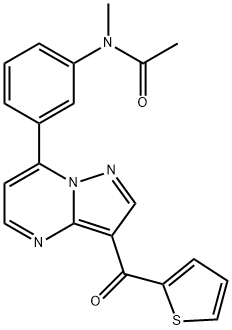
- CAS No.
- 325715-02-4
- Chemical Name:
- Indiplon
- Synonyms
- CS-779;INDIPLON;Nbi 34060;CL 285,489);Unii-8bt63da42e;Indiplon (NBI 34060;AcetaMide,N-Methyl-N-[3-[3-(2-thienylcarbonyl)pyrazolo[1,5-a]pyr;N-Methyl-N-[3-[3-[2-thienylcarbonyl]pyrazolo[1,5-a]pyrimidin-7-yl]phenyl]acetamide;N-Methyl-N-[3-[3-(thien-2-ylcarbonyl)pyrazolo[1,5-a]pyrimidin-7-yl]phenyl]acetamide;N-methyl-N-[3-[3-(thiophene-2-carbonyl)pyrazolo[1,5-a]pyrimidin-7-yl]phenyl]acetamide
- CBNumber:
- CB81011304
- Molecular Formula:
- C20H16N4O2S
- Molecular Weight:
- 376.43
- MOL File:
- 325715-02-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/26 14:13:49
Indiplon Chemical Properties,Uses,Production
Description
Indiplon is a pyrazolopyrimidine that acts as a high-
Uses
Indiplon is a member of pyrimidines with sedative, hypnotic. Indiplon has been used in trials studying the treatment of Insomnia and Depression.
Biological Activity
Potent GABA A receptor positive allosteric modulator that acts at the benzodiazepine site (K i values are 1.2 and 1.7 nM in rat frontal cortex and cerebellum respectively). Displays ~ 10-fold selectivity for α 1 subunit-containing receptors (EC 50 values are 2.6, 24, 60 and 77 nM for α 1 β 2 γ 2, α 2 β 2 γ 2, α 3 β 3 γ 2 and α 5 β 2 γ 2 receptors respectively). Exhibits sedative, hypnotic, anxiolytic and anticonvulsant activity in vivo and is orally active.
Clinical Use
Indiplon is a novel sedative-hypnotic recently approved for the treatment of insomnia. Like other non-benzodiazepine hypnotics, its mechanism of action is to modulate subunits, especially the alpha-1 subunit, of the GABA receptor complex in order to induce sedation. Indiplon was developed in two different formulations to address two different types of insomnia complaint: indiplon-IR (immediate release) was designed for sleep onset difficulties, while indiplon-MR (modified release) was developed for sleep maintenance insomnia.
Mode of action
Indiplon is a nonbenzodiazepine sedative/hypnotic that is relatively new to the marketplace. It is currently undergoing clinical trials and has been under consideration by the FDA. Caldwell et al. (2009) indicated that indiplon is chemically similar in structure to zaleplon and has a half-life of approximately 1.5 h. Indiplon, which is said to work by enhancing the action of the inhibitory neurotransmitter, y-Aminobutyric acid(GABA), is like most other nonbenzodiazepine sedatives. lt is being produced in a modified release formula that will extend its half-life to aid in sleep maintenance (Ebert et al.2006).An indiplon immediate-release version targets sleep onset insomnia, whereas a modified-release form addresses sleep maintenance insomnia. Both forms of indiplon have shown improvement compared with a placebo in patients with primary insomnia in various areas of subjective and objective sleep measurements (Lankford and Ancoli-Israel 2007; Marrs 2008).Specifically, improvements in total sleep time, latency to persistent sleep,latency to sleep onset, wake after sleep onset,and sleep quality have been noted in clinical trials.So far, trials evaluating both indiplon immediate-release and modified-release have not identified any major serious adverse effects (Marrs 2008).
Indiplon Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 | China | 3146 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29898 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | China | 9618 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10522 | 58 | Inquiry |





