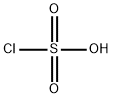
Chlorosulfonic acid
- CAS No.
- 7790-94-5
- Chemical Name:
- Chlorosulfonic acid
- Synonyms
- chlorosulphonic acid;chlorosulphuric acid;chlorosulfonic;acidechlorosulfonique;Chlorsulfonsure;AKOS BBS-00004309;CHLOROSULFONIC ACID;Sulfric chlorohydrin;Sulfurochloridicacid;Chloridosulfuric acid
- CBNumber:
- CB6270016
- Molecular Formula:
- ClHO3S
- Molecular Weight:
- 116.52
- MOL File:
- 7790-94-5.mol
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | -80°C |
|---|---|
| Boiling point | 151-152 °C/755 mmHg (lit.) |
| Density | 1.753 g/mL at 25 °C (lit.) |
| vapor density | 4 (vs air) |
| vapor pressure | 1 mm Hg ( 25 °C) |
| refractive index |
n |
| Flash point | 158°C |
| storage temp. | Room Temperature, under inert atmosphere |
| solubility | Miscible with hydrocarbons. |
| form | Oily Liquid, Fuming In Air |
| pka | -6.49±0.15(Predicted) |
| Specific Gravity | 1.753 |
| color | Yellow to brown |
| PH Range | 1 |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Merck | 14,2165 |
| Stability | Stable, but reacts violently with water. In case of spills, mop up with sand - do not add water. Reacts with most metals to yield (flammable) hydrogen gas. Incompatible with strong bases, carbonates, water, combustible materials, strong oxidizing agents, most metals, organic materials, sulfides, cyanides, carbides. |
| LogP | -0.003 at 25℃ |
| CAS DataBase Reference | 7790-94-5(CAS DataBase Reference) |
| EPA Substance Registry System | Chlorosulfonic acid (7790-94-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H330-H335 | |||||||||
| Precautionary statements | P260-P271-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 14-35-37 | |||||||||
| Safety Statements | 26-45 | |||||||||
| RIDADR | UN 1754 8/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | FX5730000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28062000 | |||||||||
| NFPA 704 |
|
Chlorosulfonic acid price More Price(2)
Chlorosulfonic acid Chemical Properties,Uses,Production
Chemical Properties
Chlorosulfonic acid, ClS03H, also known as chlorosulfuric acid and sulfuric chlorohydrin, is an colorless to light yellow oily liquid. It is formed from sulfur trioxide and hydrogen chloride, but decomposes in water to form hydrochloric acid and sulfuric acid.It is a vigorous dehydrating agent and is used in manufacturing synthetic drugs,poison gas, and saccharin.
Uses
Chlorosulfonic acid is used as detergent and as an anti-contrail agent. It is used as an intermediate in the production of other substances such as pharmaceuticals and chemicals. It finds application in producing smoke screens.
General Description
A colorless to yellow colored fuming liquid with a pungent odor. Density 14.7 lb / gal. Causes severe burns. Very toxic by inhalation. Corrosive to metals.
Reactivity Profile
Chlorosulfonic acid is a strong oxidizing acid. Reacts violently with water, strong mineral acids and bases, alcohols, finely dispersed organic matter. Dangerously incompatible with combustible materials, nitrates, chlorates, metallic powders, carbides, picrates, and fulminates. Undergoes possibly violent reactions with acetic acid, acetic anhydride, acetonitrile, acrolein, acrylic acid, acrylonitrile, alkali, allyl alcohol, allyl chloride, ammonium hydroxide, aniline, butyraldehyde, cresol, cumene, diethyleneglycol methyl ether, diisopropyl ether, diphenyl ether, ethyl acetate, ethyl acrylate, ethylene chlorohydrin, ethylenediamine, ethylene glycol, glyoxal, hydrocarbons (hexane, heptane), hydrogen peroxide, isoprene, powdered metals, methyl ethyl ketone, propylene oxide, vinyl acetate. When heated to decomposition, Chlorosulfonic acid emits toxic fumes of hydrogen chloride and oxides of sulfur [Sax, 9th ed., 1996, p. 831]. Reaction with phosphorus accelerates out of control and culminates in an explosion [Heumann, K. et al., Ber., 1882, 15, p. 417]. Mixing chlorosulfuric acid and 98% sulfuric acid may evolve HCl [Subref: Anon, Loss Prev. Bull. 1977, (013), 2-3].
Hazard
Toxic by inhalation; strong irritant to eyes and skin; causes severe burns. Can ignite combustible materials. Evolves hydrogen on contact with most metals.
Health Hazard
INHALATION: vapor extremely irritating to lungs and mucous membranes. Vapor has such a sharp and pentrating odor that inhalation of severely toxic quantities is unlikely unless it is impossible to escape the fumes. CONTACT WITH EYES OR SKIN: liquid acid will severely burn body tissue.
Safety Profile
A poison irritant. See also SULFURIC ACID. Chlorosulfonic acid is corrosive, can cause severe acid burns and is very irritating to the eyes, lungs, and mucous membranes. It can cause acute toxic effects either in the liquid or vapor state. Inhalation of concentrated vapor may cause loss of consciousness with serious damage to lung tissue. Contact of liquid with the eyes can cause severe burns if the liquid is not immediately and completely removed. It also causes severe sh burns due to its highly corrosive action. Upon ingestion it
Potential Exposure
Used to make pesticides, detergents, pharmaceuticals, dyes, resins, sulfonated oils; intermediate for dyes and pharmaceuticals; and pesticides. Although no military designation has been assigned chlorosulfonic acid may have been used as a choking/pulmonary agent
Shipping
UN1754 Chlorosulfonic acid (with or without sulfur trioxide), Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poison Inhalation Hazard, Inhalation Hazard Zone B.
Purification Methods
Distil the acid in an all-glass apparatus, taking the fraction boiling at 156-158o. It reacts EXPLOSIVELY with water [Cremlyn Chlorosulfonic acid: A Versatile Reagent, Royal Society of Chemistry UK, 2002, p 308, ISBN 0854044981, Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 385 1963].
Incompatibilities
Explosively reacts with water, forming sulfuric and hydrochloric acid and dense fumes. Dangerously reactive, avoid contact with all other material. Violent reaction with many compounds, including reducing agents; alcohols, chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur dioxide, sulfur/ friction, sulfuric acid, tungsten, hydrogen trisulfide, diphenyl ether, finely divided metals, silver nitrate. Contact with phosphorous may cause fire and explosions. Forms explosive material with ethyl alcohol. Attacks many metals; reaction with steel drums forms explosive hydrogen gas, which must be periodically relieved.
Chlorosulfonic acid Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of8
chevron_right7790-94-5(Chlorosulfonic acid)Related Search:
1of4
chevron_right




