BAF-312(SiponiMod)
- CAS No.
- 1230487-00-9
- Chemical Name:
- BAF-312(SiponiMod)
- Synonyms
- SiponiMod;Siponimod (BAF-312);SIPONIMOD; SIPONIMOD [INN]; BAF-312; BAF 312;(E)-1-(4-(1-(4-cyclohexyl-3-(trifluoromethyl)benzyloxyimino)ethyl)-2-ethylbenzyl)azetidine-3-carboxylic acid;CS-1753;Sinimod;Siponimod D11;Siponimod [INN];BAF-312(SiponiMod);Formyl sitagliptin d4
- CBNumber:
- CB62725945
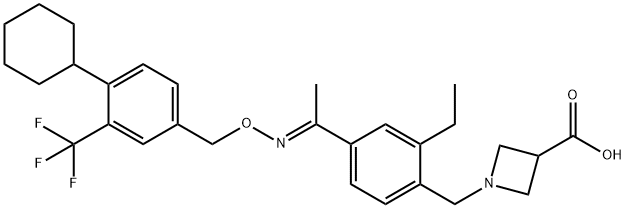
- Molecular Formula:
- C29H35F3N2O3
- Molecular Weight:
- 516.59
- MOL File:
- 1230487-00-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/17 18:22:24
| Melting point | 154 - 157°C |
|---|---|
| Boiling point | 602.0±65.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 2.69±0.20(Predicted) |
| form | Solid |
| color | White to Off-White |
| InChIKey | KIHYPELVXPAIDH-HNSNBQBZSA-N |
| SMILES | N1(CC2=CC=C(/C(=N/OCC3=CC=C(C4CCCCC4)C(C(F)(F)F)=C3)/C)C=C2CC)CC(C(O)=O)C1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| NFPA 704 |
|
BAF-312(SiponiMod) Chemical Properties,Uses,Production
Description
S1p signaling is important for immune, cardiovascular, and CNS function.Like its FDA approved predecessor fingolimod, siponimod is an oral S1p signaling modulator.While fin- golimod binds four out of five known S1p receptors, siponimod is selective for receptor sub- types 1 and 5. This selectivity may allow for an improved adverse event profile.
Uses
Siponimod is a functional sphingosine -1 phosphate (SIP) antagonist used in the treatment of multiple sclerosis and other central nervous system-related diseases in human subjects. .
Side effects
Siponimod,2 mg/day, was studied in a Phase I placebo-controlled trial in patients with secondary progressive Ms.206The primary endpoint was time to 3 months confirmed dis- ability progression. There were substantive side effects from the drug,mainly increased liver transaminase concentrations,bradycardia, and bradyarrhythmia at treatment initiation.With subsequent siponimod administration,there was an increased incidence of macular edema, hypertension,varicella zoster reactivation, and convulsions compared to placebo. By grad- ually escalating the initial dose of siponimod, cardiac first-dose effects were reduced.While the results of this trial are encouraging,long- term safety issues may be a greater concern5or since persons with secondary progressive MS as a group are older,with a higher incidence of comorbidities that could preclude use of siponimod.
BAF-312(SiponiMod) Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SIMSON PHARMA LIMITED | +91-8291074273 | Shanghai, India | 228 | 58 | Inquiry |
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| Venkatasai Life Sciences | +91-9908134868 +91-8008303069 | Hyderabad, India | 186 | 58 | Inquiry |
| Maithri Drugs Pvt Ltd | +91-9059204566 +91-9848881740 | Telangana, India | 64 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49734 | 58 | Inquiry |
| Shijiazhuang Dingmin Pharmaceutical Sciences Co., Ltd. | +86-0311-67591193 +8613931880626 | China | 256 | 58 | Inquiry |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | China | 12809 | 58 | Inquiry |
| Wuhan Haorong Biotechnology Co.,ltd | +86-18565342920; +8618565342920 | China | 305 | 58 | Inquiry |
Related articles
- The introduction of Siponimod
- Siponimod is an oral selective sphingosine 1-phosphate receptor subtypes 1 and 5 (S1PR1,5) modulator being developed by Novart....
- Jan 16,2024
1230487-00-9(BAF-312(SiponiMod))Related Search:
1of4
chevron_right




