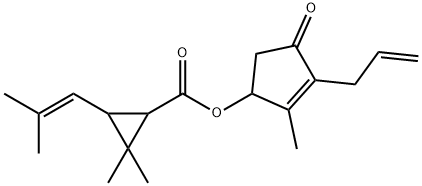
Allethrin
- CAS No.
- 584-79-2
- Chemical Name:
- Allethrin
- Synonyms
- Esbioi;fmc249;nia249;oms468;D-TRANS;exthrin;fda1446;FMC 249;NIA 249;OMS 468
- CBNumber:
- CB6681269
- Molecular Formula:
- C19H26O3
- Molecular Weight:
- 302.41
- MOL File:
- 584-79-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/9/26 17:18:59
| Melting point | approximate 4℃ |
|---|---|
| Boiling point | 160°C |
| Density | 1.01 |
| vapor pressure | 1.6×10-4 Pa (21 °C) |
| refractive index | nD20 1.5040; nD30 1.5023 |
| Flash point | 66 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform: Slightly Soluble |
| form | Viscous Liquid |
| Water Solubility | 2 mg l-1 |
| color | Clear amber |
| BRN | 2294836 |
| LogP | 4.780 |
| CAS DataBase Reference | 584-79-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Bioallethrin(584-79-2) |
| EPA Substance Registry System | Allethrin (584-79-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H332-H410 | |||||||||
| Precautionary statements | P273-P301+P312+P330-P304+P340+P312 | |||||||||
| Hazard Codes | Xn;N,N,Xn | |||||||||
| Risk Statements | 20/22-50/53-40 | |||||||||
| Safety Statements | 36-60-61-36/37-24/25-23 | |||||||||
| RIDADR | UN 3082 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GZ1925000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29183000 | |||||||||
| Toxicity | LD50 oral in rabbit: 4290mg/kg | |||||||||
| NFPA 704 |
|
Allethrin price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 33396 | Allethrin PESTANAL?, analytical standard | 584-79-2 | 100MG | ₹5964.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 33309 | Esbiothrin PESTANAL?, analytical standard | 584-79-2 | 100MG | ₹5228.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 31489 | Bioallethrin PESTANAL?, analytical standard | 584-79-2 | 250MG | ₹4481.55 | 2022-06-14 | Buy |
Allethrin Chemical Properties,Uses,Production
Chemical Properties
Yellow Oil
Uses
Allethrin is a synthetic pyrethroid derivative used as an insecticide. Allethrin is commonly used in many household insecticide products due to its low toxicity towards humans.
Definition
Generic name for 2-allyl4-hydroxtcyclopenten-1-one ester of chrysanthemummonocarboxylic acid. A synthetic insecticide structurally similar to pyrethrin and used in the same manner. For other synthetic analogs, see barthrin, cyclethrin, ethythrin, furethrin. Pyrethrin I differs in having a 2,4-pentadienyl group in place of the allyl of allethrin.
General Description
A clear amber-colored viscous liquid. Insoluble and denser than water. Toxic by ingestion, inhalation, and skin absorption. A synthetic household insecticide that kills flies, mosquitoes, garden insects, etc.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Allethrin is an ester and ketone. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Agricultural Uses
Insecticide: Allethrin is used almost exclusively to control flying and crawling insects in homes and industrial locations. Used extensively in pet animal shampoos, to treat lice in humans and in home and industrial sprays for flying insects, mosquitos, etc. It is available as mosquito coils, mats, oil formulations and as an aerosol spray. It may be hazardous to the environment; special attention should be given to fish and honey bees. Not currently registered in the U.S. Not approved for use in EU countries. Depending on CAS registry number there are probably >100 global suppliers.
Trade name
BIOALLETHRIN®; BIOALLETHRIN TECHNICAL®; d-CISALLETHRIN®; ESBIOTHRIN®; EXTHRIN® FMC 249®; NIA 249®; OMS 468®; PYNAMIN®; PYNAMIN-FORTE®; PYRESIN®; PYRESYN®; PYREXCEL®; PYROCIDE®; SBP 1382/ BIOALLETHRIN CONCENTRATE®
Contact allergens
Pyrethroids, also called pyrethrinoids, are neurotoxic synthetic compounds used as insecticides, with irritant properties. Cypermethrin and fenvalerate have been reported as causing positive allergic patch tests, but only fenvalerate was relevant in an agricultural worker.
Safety Profile
Poison by intravenous, intracerebral, and intraperitoneal routes. Moderately toxic by ingestion and skin contact. An allergen. An insecticide. It can cause liver and kidney damage by all routes of entry into the body. Lung congestion may occur due to exposure. Local contact may cause contact dermatitis. Inhalation may cause asthma, coughing, wheezing, running nose and eyes. Mutation data reported. See also ALLYL COMPOUNDS and ESTERS. Slight fire hazard. When heated to decomposition it emits acrid fumes.
Metabolic pathway
Allethrin was the first synthetic pyrethroid. Its stereochemistry is RS(cyclopentenyl)lRcis-trans. It was further developed as bioallethrin (RSlRtvuns) and then as S-bioallethrin (SlRtvuns), the most potent of the three. All are very sensitive to light and are used almost entirely indoors. Thus, there is only a limited amount of information on their environmental fate published in the literature. Information on photodegradation and on metabolism in insects and rodents has been reported.
Allethrin Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shogun Organics Ltd | +91-2266776846 +91-2266776845 | Maharashtra, India | 8 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Vallabh Pesticides Limited | 08048372724Ext 120 | Gujarat, India | 5 | 58 | Inquiry |
| M/S Ahmad Beez Store | 08048250244Ext 776 | Uttar Pradesh, India | 1 | 58 | Inquiry |
| Solex Chemicals Private Limited | 08068979890Ext 189 | Kolkata, India | 1 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | China | 2989 | 55 | Inquiry |





