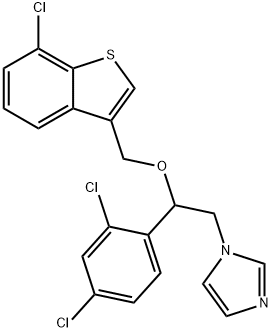
Sertaconazole nitrate
- CAS No.
- 99592-32-2
- Chemical Name:
- Sertaconazole nitrate
- Synonyms
- SERTACONAZOLE;eno;Zalain;FI-7056;fi-7045;Dermofix;Sertaconazol;Sertaconazole API;Sertaconazole nitrate;Sertaconazole nitrate USP/EP/BP
- CBNumber:
- CB6701774
- Molecular Formula:
- C20H15Cl3N2OS
- Molecular Weight:
- 437.77
- MOL File:
- 99592-32-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/8/28 18:13:45
| Melting point | 146-147° |
|---|---|
| Boiling point | 614.1±55.0 °C(Predicted) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 6.68±0.12(Predicted) |
| color | White to Pale Yellow |
| CAS DataBase Reference | 99592-32-2(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H335-H315 |
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P |
Sertaconazole nitrate Chemical Properties,Uses,Production
Description
Sertaconazole has been developed and launched for the treatment of dermatological fungal infections by Ferrer Internacional S. A. Mylan received FDA approval for sertaconazole nitrate cream for the treatment of athlete's foot (tinea pedis) at the end of 2003.
Uses
An imidazole antifungal agent, inhibits the synthesis of ergosterol, an essential cell wall component of fungi.
Indications
Topical treatment of mycoses of the skin induced or sustained by fungi such as yeasts and dermatophytes. New formulations for the treatment of vaginal mycoses are in development.
Antimicrobial activity
Sertaconazole is a rather new broad-spectrum imidazole antimycotic with activity against almost all species of pathogenic fungi. It also has excellent activity against pathogenic yeasts.
Synthesis
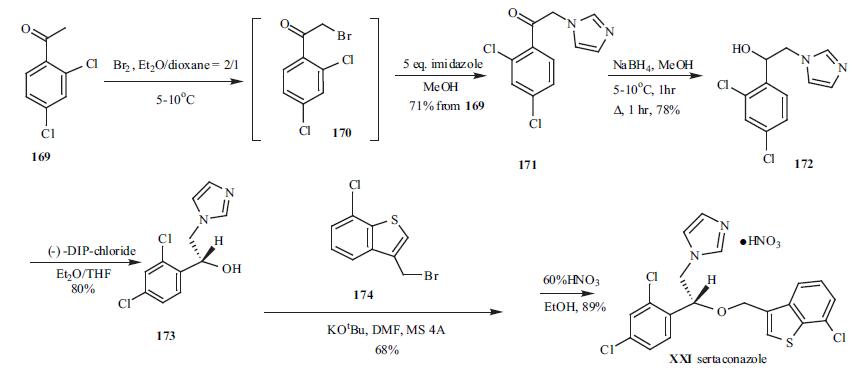
2,4-Dichloro acetophenone 169 was brominated at low temperature to give bromide intermediate 170, which was used without isolation. To the same pot, five-fold excess of imidazole was added to give imidazolylacetophenone 171 in 71% yield from 169. Sodium borohydride was employed to reduce ketone 171 to alcohol 172 in 78% yield. Racemic alcohol 172 was resolved with (-)-DIP-chloride to give its corresponding chiral R-alcohol 173 in 80% yield. Compound 173 was then alkylated with 3-bromomethyl-7-chlorobenzo[b]thiophene (174) in dry DMF in the presence of potassium t-butoxide to give the alkylation product in 68% yield. Finally, 60% nitric acid was used to make sertaconazole mononitrate (XXI) in 89% yield.
Solubility in organics
Fairly soluble in ethanol (1.7 %), chloroform (1.5 %); slightly soluble in acetone (0.95 %); very slightly soluble in noctanol (0.069 %). Practically insoluble in water (< 0.01 %).
Sertaconazole nitrate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SURYA LIFE SCIENCES LIMITED | +91-2646226290 +91-9428511112 | Gujarat, India | 62 | 58 | Inquiry |
| SEUTIC | +91-8309787199 +91-8309787199 | Hyderabad, India | 124 | 58 | Inquiry |
| Sekhment pharmaventures | +91-9030088669 +91-9030088669 | Mumbai, India | 105 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Chempifine Chemicals | +91-2225667766 +91-2225667766 | Punjab, India | 144 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Bioaltus Laboratories Pvt Ltd | +91-2249744539 +91-9820139885 | Maharashtra, India | 34 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
Related articles
- Mechanism of action of Sertaconazole
- Sertaconazole contains a benzothiophene group that both augments its antifungal activity and confers a highly lipophilic fragm....
- Mar 30,2022
99592-32-2(Sertaconazole nitrate)Related Search:
1of4
chevron_right




