Cupric oxide
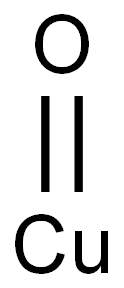
- CAS No.
- 1317-38-0
- Chemical Name:
- Cupric oxide
- Synonyms
- CuO;COPPER OXIDE;COPPER(II) OXIDE;cupric;Cupric oxide powder;COPPER(II) OXIDE (CUO);COPPER MONOXIDE;Copper(II) oxide, 99.999%;Copper(II) oxide, 99.9999% (metals basis);Copper(II) oxide, Puratronic(R), 99.995% (metals basis)
- CBNumber:
- CB6853040
- Molecular Formula:
- CuO
- Molecular Weight:
- 79.55
- MOL File:
- 1317-38-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/5 13:26:43
| Melting point | 1326 °C |
|---|---|
| Density | 6.315 |
| refractive index | 2.63 |
| storage temp. | no restrictions. |
| solubility | Aqueous Acid (Slightly), Methanol (Slightly) |
| form | powder |
| color | Brown to black |
| Specific Gravity | 6.3-6.49 |
| Odor | at 100.00?%. odorless |
| PH | 7 (50g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Merck | 14,2646 |
| Dielectric constant | 18.1(Ambient) |
| Exposure limits |
ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.1 mg/m3; TWA 1 mg/m3 |
| Stability | Stable. Incompatible with reducing agents, hydrogen sulfide, aluminium, alkali metals, finely powdered metals. |
| CAS DataBase Reference | 1317-38-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Copper(ii) oxide(1317-38-0) |
| EPA Substance Registry System | Cupric oxide (1317-38-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H410 | |||||||||
| Precautionary statements | P273-P391-P501 | |||||||||
| Hazard Codes | Xn,Xi,N | |||||||||
| Risk Statements | 22-37-50 | |||||||||
| Safety Statements | 22-36-61-60-29 | |||||||||
| RIDADR | UN 3077 9 / PGIII | |||||||||
| OEB | D | |||||||||
| OEL | TWA: 0.1 mg/m3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GL7900000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| HS Code | 28255000 | |||||||||
| Toxicity | LD50 orally in Rabbit: 470 mg/kg | |||||||||
| IDLA | 100 mg Cu/m3 | |||||||||
| NFPA 704 |
|
Cupric oxide price More Price(73)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 544868 | Copper(II) oxide nanopowder, <50?nm particle size (TEM) | 1317-38-0 | 5G | ₹2980 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 792004 | Copper(II) oxide nanorod, diam. × L 10-12?nm × 75-100?nm | 1317-38-0 | 5G | ₹11149.75 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 544868 | Copper(II) oxide nanopowder, <50?nm particle size (TEM) | 1317-38-0 | 25G | ₹9760 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 450812 | Copper(II) oxide powder, 99.99% trace metals basis | 1317-38-0 | 25G | ₹18629.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 450812 | Copper(II) oxide powder, 99.99% trace metals basis | 1317-38-0 | 100G | ₹44642.3 | 2022-06-14 | Buy |
Cupric oxide Chemical Properties,Uses,Production
Description
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.Mainly used in wood preservatives, ceramics, and mineral supplements for animal feed.
Copper(II) oxide nanoparticles (NPCuO) have industrial applications as antimicrobial agents in textiles and paints, and catalysts in organic synthesis. They may also be produced from electronic wastes. Cupric oxide poses potential health and environmental concern due to toxic and mutagenic particles generating reactive oxygen species.
Chemical Properties
Black fine free powder
Uses
Cupric Oxide can be used as a dietary ingredient and as a nutrient. Copper aids in the absorption of iron, in the formation of red blood cells and the proper bone formation and maintenance.
As pigment in glass, ceramics, enamels, porcelain glazes, artificial gems; in manufacture of rayon, other Cu Compounds; in sweetening petroleum gases; in galvanic electrodes; as flux in metallurgy; in correcting Cu deficiencies in soil; as optical-glass polishing agent; in antifouling paints, pyrotechnic compositions; as exciter in phosphor mixtures; as catalyst for organic reactions; in high tempereture superconductors.
Definition
A black solid prepared by the action of heat on copper(II) nitrate, hydroxide, or carbonate. It is a basic oxide and reacts with dilute acids to form solutions of copper(II) salts. Copper(II) oxide can be reduced to copper by heating in a stream of hydrogen or carbon monoxide. It can also be reduced by mixing with carbon and heating the mixture. Copper(II) oxide is stable up to its melting point, after which it decomposes to give oxygen, copper(I) oxide, and eventually copper.
Reactions
Copper(II) oxide dissolves in mineral acids such as hydrochloric acid, sulfuric acid or nitric acid to give the corresponding copper(II) salts:
CuO + 2 HNO3 → Cu(NO3)2 + H2O
CuO + 2 HCl → CuCl2 + H2O
CuO + H2SO4 → CuSO4 + H2O
It reacts with concentrated alkali to form the corresponding cuprate salts:
2 MOH + CuO + H2O → M2[Cu(OH)4]
It can also be reduced to copper metal using hydrogen, carbon monoxide, or carbon:
CuO + H2 → Cu + H2O
CuO + CO → Cu + CO2
2 CuO + C → 2Cu + CO2
When cupric oxide is substituted for iron oxide in thermite the resulting mixture is a low explosive, not an incendiary.
benefits
Cupric oxide is an oxide of the mineral copper. It is an essential element needed by the body to perform a host of functions.
Cupric oxide is used by specific enzymes to help in the production of energy, to create collagen and elastin, to metabolize iron, and in many functions of the brain and central nervous system. Cupric oxide is found in health supplements such as vitamins and health aid treatments.
Copper is a mineral that is needed in the body in small doses but has the ability to become toxic at high levels. Additional supplements of copper beyond what you should get in your normal diet should be discussed with a doctor.
General Description
Copper oxides (Cu2O, CuO) are p-type semiconductor materials with small band gap energy. High physical and chemical stability of metal oxide nanoparticles renders them extremely useful in catalytic applications.The structures of the compounds are monoclinic. Nanoscaled copper oxide compounds can be prepared by thermal plasma technology. A study reports its antimicrobial properties.
Health Hazard
Exposures to copper fume cause fever, chills, muscle aches, nausea, dry throat, coughing, weakness, lassitude, irritation to the eyes, nose, throat, skin, upper respiratory tract, chest tightness, nose bleed, edema, and lung damage. Symptoms of copper fume poisoning also include metallic or sweet taste, skin itching, skin rash, skin allergy, and a greenish color to the skin, teeth, and hair. Workers have increased risk of Wilson’s disease.
Precautions
Occupational workers should use protective clothing, such as suits, gloves, footwear, and headgear, and promptly change the contaminated clothing/work dress. Workers should not eat, smoke, or drink where copper dust or powder is handled, processed, or stored. Workers should wash hands carefully before eating, drinking, smoking, or using the toilet. The workplace should have a vacuum or a wet method facility to reduce the metal dust during cleanup
Cupric oxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| MEGHACHEM INDUSTRIES | +91 9825032614 | Ahmedabad, India | 11 | 58 | Inquiry |
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Yogi Dye Chem Industries | +91-9821098577 +91-9821098577 | Mumbai, India | 124 | 58 | Inquiry |
| CHEMORGE JAIN | +91-9727716206 +91-9727716206 | Ahmedabad, India | 9 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Parikh Enterprises Pvt Ltd | +91-7922871471 +91-9925000571 | Gujarat, India | 10 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Perry Chem | +91-9898110710 +91-9374177467 | Gujarat, India | 3 | 58 | Inquiry |
| Jagannath Chemicals | +91-9443123685 +91-9047021198 | Tamil Nadu, India | 2 | 58 | Inquiry |
| Shyam Chemicals Pvt Ltd | +91-9820028842 +91-9820028842 | Mumbai, India | 10 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| MEGHACHEM INDUSTRIES | 58 |
| ADVENT CHEMBIO PRIVATE LIMITED | 58 |
| Yogi Dye Chem Industries | 58 |
| CHEMORGE JAIN | 58 |
| JSK Chemicals | 58 |
| Parikh Enterprises Pvt Ltd | 58 |
| Merck Ltd | 58 |
| Perry Chem | 58 |
| Jagannath Chemicals | 58 |
| Shyam Chemicals Pvt Ltd | 58 |
Related articles
- Synthesis of CuO Nanoparticles
- Cupric oxide is an inorganic chemical compound composed of cuprous ion and oxide ion. Cupric cuprous are the two forms of copp....
- Dec 20,2021
1317-38-0(Cupric oxide)Related Search:
1of4
chevron_right




