LEPTOMYCIN A
- CAS No.
- 87081-36-5
- Chemical Name:
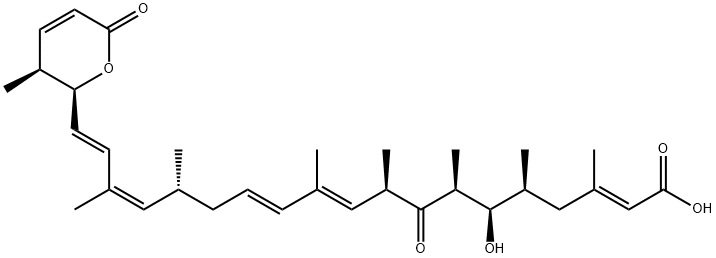
- LEPTOMYCIN A
- Synonyms
- PD-118607;NSC 369326;Antibiotic PD-118607;Antibiotic ATS-1287A;LEPTOMYCIN A USP/EP/BP;QECBVZBMGUAZDL-DLWOFZAMSA-N;ATS 1287A, PD 118607, JildaMycin;19-(3,6-Dihydro-3-methyl-6-oxo-2H-pyran-2-yl)-6-hydroxy-3,5,7,9,11,15,17-heptamethyl-8-oxo-2,10,12,16,18-nonadecapentaenoic acid;19-(3,6-dihydro-3-methyl-6-oxo-2h-pyran-2-yl)-3,5,7,9,11,15,17-heptamethyl-6-hydroxy-8-oxo-2,10,12,16,18-nonadecapentaenoic acid;2,10,12,16,18-Nonadecapentaenoicacid,19-[(2S,3S)-3,6-dihydro-3-methyl-6-oxo-2H-pyran-2-yl]-6-hydroxy-3,5,7,9,11,15,17-heptamethyl-8-oxo-,(2E,5S,6R,7S,9R,10E,12E,15R,16Z,18E)-
- CBNumber:
- CB6972730
- Molecular Formula:
- C32H46O6
- Molecular Weight:
- 526.7
- MOL File:
- 87081-36-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:14
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H301+H311+H331-H370 | |||||||||
| Precautionary statements | P210-P260-P280-P301+P310-P311 | |||||||||
| Hazard Codes | F,T | |||||||||
| Risk Statements | 11-23/24/25-39/23/24/25 | |||||||||
| Safety Statements | 16-36/37-45 | |||||||||
| RIDADR | UN 1230 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| HS Code | 3822000000 | |||||||||
| NFPA 704 |
|
LEPTOMYCIN A Chemical Properties,Uses,Production
Description
Leptomycin A is an inhibitor of CRM1 (exportin 1) that blocks CRM1 interaction with nuclear export signals, preventing the nuclear export of a broad range of proteins. Leptomycin A inhibits Rev translocation, though less potently than leptomycin B with IC50 values of 0.8 and 0.1 nM, respectively after 7 hours. Rev export to the cytoplasm is required for HIV-1 replication. Leptomycin A was originally identified as an antifungal agent.
Uses
Leptomycin A, a methyl analogue of leptomycin B, is a minor component of the leptomycin complex produced by selected Streptomyces species. Leptomycin B is a potent inhibitor of the nuclear transport receptor CRM1. Leptomycin A is also likely to inhibit nuclear transport and may offer differences in exporter selectivity. Leptomycin A shows antimicrobial activity against Schizosaccaromyces pombe and Mucor rouxianus.
Definition
ChEBI: A leptomycin having all-trans double bonds and a seventh methyl substituent at position 17.
LEPTOMYCIN A Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | China | 2316 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Shanghai Minbiotech Co., Ltd. | +8617315815539 | CHINA | 129 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | China | 3136 | 58 | Inquiry |
| Wuhan Jingkang en Biomedical Technology Co., Ltd | +8613720134139 | China | 4692 | 58 | Inquiry |
| TargetMol Chemicals Inc. | 15002134094 | China | 28118 | 58 | Inquiry |
87081-36-5(LEPTOMYCIN A)Related Search:
1of2
chevron_right




