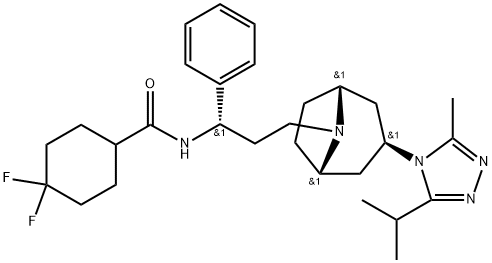
Maraviroc
- CAS No.
- 376348-65-1
- Chemical Name:
- Maraviroc
- Synonyms
- CS-49;Celsentri;maroviroc;Maraviroc;634 Hexilure;Maraviroc API;Maraviroc, >=99%;Maraviroc(UK427857);Maraviroc USP/EP/BP;Maraviroc(Selzentry)
- CBNumber:
- CB71270957
- Molecular Formula:
- C29H41F2N5O
- Molecular Weight:
- 513.68
- MOL File:
- 376348-65-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/7 16:07:22
| Melting point | 79-81°C |
|---|---|
| Density | 1.29±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: >30mg/mL |
| pka | 7.3(at 25℃) |
| form | white powder |
| color | White |
| optical activity | [α]-15/D |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| InChIKey | GSNHKUDZZFZSJB-RWJISDSDNA-N |
| SMILES | C(N1[C@@H]2CC[C@H]1C[C@H](N1C(=NN=C1C(C)C)C)C2)C[C@@H](C1C=CC=CC=1)NC(C1CCC(F)(F)CC1)=O |&1:2,5,7,19,r| |
| CAS DataBase Reference | 376348-65-1(CAS DataBase Reference) |
Maraviroc price More Price(2)
Maraviroc Chemical Properties,Uses,Production
Description
Maraviroc is the first CCR5 receptor antagonist that has been developed and launched for the treatment of HIV-1. Maraviroc binds in a slowly reversible, allosteric manner to CCR5, which is one of two principle chemokine co-receptors for viral entry into the host cell, the other being CXCR4. Binding of maraviroc to CCR5 induces conformational changes within the chemokine receptor, thereby preventing CCR5 binding to the viral gp120 protein and the ultimate CCR5- mediated virus-cell fusion that is a prerequisite for HIV invasion. Maraviroc, with its unique mechanism of action as a fusion inhibitor, joins the greater than 20 marketed antiretrovirals, including nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and PIs. It is approved for use in combination with these other antiretroviral drugs in adult patients with R5-tropic HIV-1 infection (but not X4 or dual/mixed tropic HIV-1).
Chemical Properties
Brown Solid
Uses
Maraviroc is a CCR5 antagonist for MIP-1α, MIP-1β and RANTES with IC50 of 3.3 nM, 7.2 nM and 5.2 nM, respectively
Definition
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 4,4-difluorocyclohexanecarboxylic acid and the primary amino group of (1S)-3-[(3-exo)-3-(3-isopropyl-5-methyl-4H-1,2,4- riazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropylamine. An antiretroviral drug, it prevents the interaction of HIV-1 gp120 and chemokine receptor 5 (CCR5) necessary for CCR5-tropic HIV-1 to enter cells.
Acquired resistance
In most patients (c. 60%) failure of response is associated with the selection of virus that can use CXCR4 as its entry co-receptor. Evidence for the selection of virus that continues to use CCR5 has also been described.
Pharmaceutical Applications
A spirodiketopiperazine formulated as tablets for oral use.
Pharmacokinetics
Oral absorption: c. 33% (300 mg dose)
Cmax 150 mg twice daily: c. 332 μg/L*
Cmin 150 mg twice daily: c. 101 μg/L*
Plasma half-life: c. 13.2 h (30 mg iv
administration)
Volume of distribution: c. 194 L
Plasma protein binding: c. 76%
Absorption
The absolute bioavailability of a 100 mg dose is 23% and is predicted to be 33% after a 300 mg dose. Co-administration of a 300 mg tablet and a high-fat meal has resulted in reduced Cmax and AUC by 33% in healthy volunteers. However, because no food restrictions were enacted during clinical trials, maraviroc may be taken with or without food.
Distribution
Animal experiments suggest low CSF concentrations around 10% of free plasma concentrations. It is not known whether it passes into breast milk. A study of genital tract secretions and vaginal tissue in healthy HIV-uninfected female volunteers suggest a concentration in cervicovaginal fluid more than four-fold higher than that in plasma.
Metabolism
It is a substrate for CYP3A4 and P-glycoprotein, but does not appear to inhibit or induce CYP3A4.
Excretion
Seventy-six and 19% of a radiolabeled maraviroc dose were recovered in the feces and urine, respectively.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
in treatment-experienced patients
On November 20, 2009, the US Food and Drug Administration approved
a supplemental new drug application to expand the indication for
maraviroc to include combination antiretroviral treatment of treatmentnaive
adults infected with CCR5-tropic HIV virus
Side effects
Overall, maraviroc was well tolerated with the most common adverse events being cough, fever, colds, rash, muscle and joint pain, stomach pain, and dizziness. While some patients did experience liver enzyme elevation, these events did not appear to be doserelated. Since hepatotoxicity did occur in one patient with prior liver function abnormalities, maraviroc s label warns of a potentially increased risk of hepatoxicity with treatment. Postural hypotension was also observed in a dosedependent manner; however, no patients discontinued therapy as a result. As a substrate for CYP3A4, the dose of maraviroc should be reduced by 50% in the presence of strong CYP3A4 inhibitors. Conversely, concomitant use of strong CYP3A4 inducers requires a 50% increase in maraviroc dose. While there are no contraindications, maraviroc should be used with caution in patients with liver dysfunction, high risk of cardiovascular events, and pre-existing postural hypotension.
Maraviroc Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Aurona Med Pharma Private Limited | +91-9618434204 +91-8074941863 | Hyderabad, India | 24 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| Cohance Lifesciences (Previously RA Chem Pharma Ltd) | +91-4044758595 +91-4044758595 | Telangana, India | 47 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| HETERO LABS LTD | (630) 931-6800 | New Delhi, India | 97 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Manus Aktteva | +91 (79) 6512-3395 | New Delhi, India | 581 | 34 | Inquiry |
Related articles
- Side effects of Maraviroc
- Maraviroc was synthesized by Pfizer Inc as UK-427,857 (4, 4-difluoro- N-[(1S)-3-[exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-....
- Apr 2,2022






