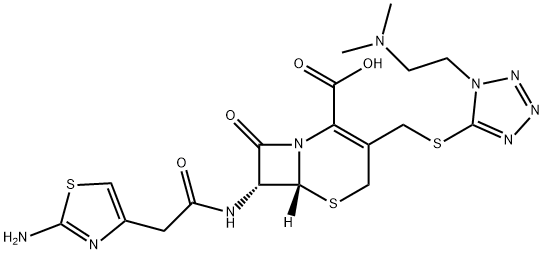
Cefotiam
- CAS No.
- 61622-34-2
- Chemical Name:
- Cefotiam
- Synonyms
- SCE 963;Texodil;CEFOTIAM;Taketiam;cgp14221e;Pansporin T;CEFOTIAM HCL;(6r-trans)--oxo;Cefotiam USP/EP/BP;Pansporin:Pansporine
- CBNumber:
- CB7367629
- Molecular Formula:
- C18H23N9O4S3
- Molecular Weight:
- 525.63
- MOL File:
- 61622-34-2.mol
- Modify Date:
- 2024/4/12 23:00:59
| Boiling point | 843℃ |
|---|---|
| Density | 1.80±0.1 g/cm3(Predicted) |
| RTECS | XI0366000 |
| Flash point | >110°(230°F) |
| storage temp. | 2-8°C |
| pka | 2.57±0.50(Predicted) |
| Water Solubility | Soluble |
| CAS DataBase Reference | 61622-34-2(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P261-P285-P304+P341-P342+P311-P501 |
Cefotiam Chemical Properties,Uses,Production
Uses
Antibacterial
Definition
ChEBI: A cephalosporin with ({1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl}sulfanyl)methyl and (2-amino-1,3-thiazol-4-yl)acetamido substituents at positions 3 and 7, respectively, of the cephem skeleton. A third generation beta-lactam cephalospo in antibiotic, it is active against a broad spectrum of both Gram positive and Gram negative bacteria.
Antimicrobial activity
A semisynthetic cephalosporin formulated as the dihydrochloride
for injection and as a prodrug ester, cefotiam hexetil, for
oral administration. Activity is similar to that of cefuroxime,
but it is somewhat more active against a range of enterobacteria
.
A 30-min intravenous infusion of the dihydrochloride
produces a peak serum concentration of 35 mg/L; the corresponding
concentration after a 1 g intramuscular dose is
17 mg/L. Oral absorption of the hexetil ester is around 65%.
Food delays absorption of the ester. The plasma half-life is
0.6–1.1 h. Around 40% is bound to plasma protein.
Urinary excretion is almost complete 4 h after the end
of intravenous infusion, but only 50–67% is recovered
unchanged; there is substantial non-renal elimination and
some evidence of saturation of renal tubular excretion at
doses above 2 g. In anuria the plasma elimination half-life
rises to 13 h and plasma and renal clearances parallel creatinine
clearance. A small amount is excreted in bile. In patients
with cholelithiasis given 0.5 or 1 g intravenously, mean concentrations
in gallbladder bile and gallbladder wall 30 min
after the dose were around 17 and 32 mg/L, respectively. In
patients with normal liver function, hepatic bile concentrations
can exceed 1 g/L.
It is generally well tolerated and has been used successfully
to treat lower respiratory infections, skin and soft-tissue infection.
It is not widely used, but is available in Japan and some
other countries.
Cefotiam Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | China | 1142 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29897 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
Related articles
- Cefotiam: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
- Cefotiam is widely used as perioperative prophylaxis in Japan. It is predominantly used intravenously (in the form of cefotiam....
- Mar 23,2022
61622-34-2(Cefotiam)Related Search:
1of4
chevron_right




