Topiramate
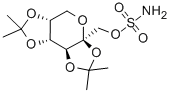
- CAS No.
- 97240-79-4
- Chemical Name:
- Topiramate
- Synonyms
- TopiraMate API;Topina;TOPAMAX;Topimax;EpitoMa;TopaMac;TopoMax;MCN 4853;EpitoMax;RWJ 17021
- CBNumber:
- CB7402630
- Molecular Formula:
- C12H21NO8S
- Molecular Weight:
- 339.36
- MOL File:
- 97240-79-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/23 13:57:25
| Melting point | 125°C |
|---|---|
| alpha | D23 -34.0° (c = 0.4 in methanol) |
| Boiling point | 438.7±55.0 °C(Predicted) |
| Density | 1.336±0.06 g/cm3(Predicted) |
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: ~44 mg/mL |
| form | solid |
| pka | 9.22±0.70(Predicted) |
| color | white |
| Water Solubility | 9.705g/L(temperature not stated) |
| Merck | 14,9547 |
| BCS Class | 3 |
| LogP | 2.970 (est) |
| EPA Substance Registry System | .beta.-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, 1-sulfamate (97240-79-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,T,F | |||||||||
| Risk Statements | 36/37/38-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 26-36-45-36/37-16-7 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LS7083000 | |||||||||
| HS Code | 29350090 | |||||||||
| Toxicity | LD50 intraperitoneal in rat: > 1500mg/kg | |||||||||
| NFPA 704 |
|
Topiramate price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T0575 | Topiramate ≥98% (HPLC), solid | 97240-79-4 | 10MG | ₹11842.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T0575 | Topiramate ≥98% (HPLC), solid | 97240-79-4 | 50MG | ₹52945.08 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T-039 | Topiramate solution 1.0?mg/mL in methanol, ampule of 1?mL, certified reference material, Cerilliant? | 97240-79-4 | 1ML | ₹10041.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1600 | Topiramate Pharmaceutical Secondary Standard; Certified Reference Material | 97240-79-4 | 1G | ₹12048.23 | 2022-06-14 | Buy |
| TCI Chemicals (India) | T2755 | Topiramate | 97240-79-4 | 1G | ₹9800 | 2022-05-26 | Buy |
Topiramate Chemical Properties,Uses,Production
Description
Topiramate, a novel sulfamate-substituted D-fructose derivative, was launched in the United Kingdom as an adjunct therapy for use in partial seizures with or without secondary generalized seizures in adult patients inadequately controlled on conventional antiepileptics. Topiramate is structurally distinct from other available antiepileptics and functions through a unique combination of several mechanisms. It appears to act by blocking voltage-sensitive sodium channels to raise the action potential threshold and block the spread of seizure, enhancing GABA activity at postsynaptic GABA receptors and reducing glutamate activity at postsynaptic AMPAtype receptors, and is also a carbonic anhydrase inhibitor. Topiramate is orally active with rapid absorption, high bioavailability, and long duration of action. Excellent efficacy has been reported as an add-on therapy in epilepsy and it is also being evaluated as a monotherapy.
Chemical Properties
White-to-Off-White Crystalline Powder
Uses
Topiramate may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations using high-performance liquid chromatography technique and spectrophotometric technique.
Definition
ChEBI: A hexose derivative that is 2,3:4,5-di-O-isopropylidene-beta-D-fructopyranose in which the hydroxy group has been converted to the corresponding sulfamate ester. It blocks voltage-dependent sodium channe s and is used as an antiepileptic and for the prevention of migraine.
Biological Functions
Topiramate is most useful in patients with generalized tonic–clonic seizures and those with partial complex seizures. Topiramate causes a higher incidence of CNSrelated side effects (primarily cognitive slowing and confusion) than other AEDs. It does not appear to cause a significant incidence of rashes or other hypersensitivity reactions; however, a significantly higher incidence of kidney stones has been observed in persons receiving topiramate than in a similar untreated population.
General Description
TPM is a sulphamate-substituted monosaccharide, a derivativeof the naturally occurring sugar D-fructose thatexhibits broad and potent AED actions at both glutamateand GABA receptors.19 It has good oral bioavailability of85% to 95%, most likely resulting from its structural similarityto D-glucose. Thus, it may be actively transportedinto the brain by the D-glucose transporter. (Recall thatD-fructose and D-glucose have identical stereochemistry atmany of their chiral centers.) Only about 20% of the drugis eliminated by hepatic metabolism (CYP2C19), the remainingdrug is excreted unchanged by the kidneys.57 Thesulphamate ester is hydrolyzed by sulfatases to the correspondingprimary alcohol, which is further oxidized to thecorresponding carboxylic acid. Even though there are noreports of significant interactions between TPM and otherAEDs, TPM is said to have a weak carbonic anhydrase inhibitoryactivity because of the presence of the sulphamatemoiety. Thus, concomitant use of TPM with other carbonicanhydrase inhibitors should be avoided.57 The exact mechanismof actions are still unknown, but TPM appears toblock glutamate release, antagonize glutamate kainicacid/AMPA receptors, and increase GABAergic transmissionby binding to a site distinct from BZDs or barbiturateson the GABAA receptor complex.
Biological Activity
Anticonvulsant. Antagonizes GluR5 kainate receptors (IC 50 = 0.46 μ M), acts as a positive allosteric modulator of GABA A receptor-mediated currents, inhibits Na v channels (IC 50 = 48.9 μ M) and inhibits L-type Ca 2+ channels. Also inhibits carbonic anhydrase (CA) (K i values are 0.1 and 0.2 μ M at rat CA II and CA IV respectively), which lowers intracellular neuronal pH.
Mechanism of action
The mechanism of action for topiramate is unknown, but several actions are thought to contribute to its AED activity. It blocks repetitive firing by acting on sodium channels, may enhance GABAA-mediated chloride flux, and appears to be an antagonist at the AMPA and KA receptors, thus blocking the effect of glutamate. In addition, recent evidence suggests inhibition of L-type calcium currents.
Pharmacokinetics
Topiramate is rapidly absorbed, with at least an 80 to 95% oral bioavailability that is unaffected by food. Following an oral dose
of topiramate, peak plasma concentration is reached in 1 to 4 hours, exhibiting linear pharmacokinetics. Protein binding is
minimal (<20%), and the usual elimination half-life is 20 to 30 hours, allowing a twice-daily dosing regimen. In the absence of
enzyme-inducing drugs, approximately 70 to 80% of the drug is excreted unchanged in the urine, with the remainder as
metabolites resulting from oxidation and hydrolysis. Enzyme-inducing AEDs alter the pharmacokinetics of topiramate by
reducing its plasma levels and increasing its rate of elimination.
In children from 4 to 17 years of age, topiramate exhibits linear pharmacokinetics, with a 50% increase in clearance rate
compared to adults. Topiramate may require up to a 50% dose reduction in patients with renal insufficiency, and a replacement dose may be needed after renal dialysis. Topiramate has demonstrated teratogenicity in animal studies.
Clinical Use
Topiramate is a sulfamate-substituted monosaccharide derived from fructose with a broad spectrum of AED activity. It is approved for monotherapy or as an adjunct drug for partial or primary generalized tonic-clonic seizures in patients older than 10 years, as adjunct therapy in children aged from 2 to 10 years with partial-onset seizures, and in persons older than 2 years with Lennox-Gastaut syndrome. Topiramate also is approved for the prophylaxis of migraine headaches.
Side effects
Common CNS side effects associated with topiramate therapy include drowsiness, dizziness, impaired concentration and
memory, speech and language difficulties, and confusion. These effects develop during the first weeks of therapy and may
decline over time. Acute closed-angle glaucoma caused by topiramate requires immediate evaluation. Only rare hepatic or
bone marrow effects have been noted thus far; however, an increased incidence of renal stones is troublesome and probably
related to the drug's activity as a carbonic anhydrase inhibitor, reducing citrate excretion and increasing urinary pH. Use of
additional carbonic anhydrase inhibitors, a ketogenic diet, or a family history of nephrolithiasis may be considered as
contraindications for using topiramate.
Topiramate is not devoid of potential interaction properties: It induces CYP3A4 and inhibits CYP2C19, thus significantly increasing plasma phenytoin levels. Topiramate also may decrease the effectiveness of oral contraceptives.
Topiramate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Venture Pharmaceuticals pvt ltd | +91-9173909075 +91-9173909075 | Gujarat, India | 45 | 58 | Inquiry |
| Sun Pharmaceutical Industries Ltd | +91-2242244224 +91-2243244324 | Maharashtra, India | 108 | 58 | Inquiry |
| KRS Pharmaceuticals Pvt. Ltd. | +91-4023176686 +91-9848996298 | Hyderabad, India | 36 | 58 | Inquiry |
| SNJ Labs Pvt Ltd | +91-9033216399 +91-9824296655 | Gujarat, India | 17 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Vasoya Industries Pvt Ltd | +91-9898083583 +91-9898083583 | Gujarat, India | 42 | 58 | Inquiry |
| Apotex Pharmachem India Pvt Ltd | +91-8022891034 +91-8022891000 | Karnataka, India | 109 | 58 | Inquiry |
| Glenmark Pharmaceuticals Limited | +912240189999 | Maharashtra, India | 93 | 58 | Inquiry |





