Furazolidone
- CAS No.
- 67-45-8
- Chemical Name:
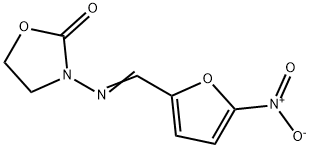
- Furazolidone
- Synonyms
- Furazolidon;Furoxane;3-(((5-Nitro-2-furanyl)methylene)amino)-2-oxazolidinone;Furoxone;Furazolidine;N-(5-NITRO-2-FURFURYLIDENE)-3-AMINO-2-OXAZOLIDIN-2-ONE;3-[[(5-Nitrofuran-2-yl)methylene]amino]oxazolidin-2-one;Furox;Neftin;NF 180
- CBNumber:
- CB8452405
- Molecular Formula:
- C8H7N3O5
- Molecular Weight:
- 225.16
- MOL File:
- 67-45-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/7 16:58:36
| Melting point | 254-256°C (dec.) |
|---|---|
| Boiling point | 366.66°C (rough estimate) |
| Density | 1.5406 (rough estimate) |
| refractive index | 1.7180 (estimate) |
| Flash point | 2 °C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | formic acid: soluble50mg/mL |
| pka | -1.98±0.20(Predicted) |
| form | powder |
| color | yellow |
| λmax | 365nm(DMSO)(lit.) |
| Sensitive | Light Sensitive |
| Merck | 14,4300 |
| BRN | 8317414 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | PLHJDBGFXBMTGZ-UITAMQMPSA-N |
| IARC | 3 (Vol. 31, Sup 7) 1987 |
| NIST Chemistry Reference | Furazolidone(67-45-8) |
| EPA Substance Registry System | Furazolidone (67-45-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361 | |||||||||
| Precautionary statements | P280 | |||||||||
| Hazard Codes | Xn,F | |||||||||
| Risk Statements | 62-40-36-20/21/22-11-68 | |||||||||
| Safety Statements | 36-22-36/37-16 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RQ3675000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
Furazolidone price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | F9505 | Furazolidone | 67-45-8 | 10G | ₹6581.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | F9505 | Furazolidone | 67-45-8 | 25G | ₹8692.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR2598 | Furazolidone Pharmaceutical Secondary Standard; Certified Reference Material | 67-45-8 | 500MG | ₹16995.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | F9505 | Furazolidone | 67-45-8 | 100G | ₹23576.85 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 46297 | Furazolidone VETRANAL?, analytical standard | 67-45-8 | 250MG | ₹5596.53 | 2022-06-14 | Buy |
Furazolidone Chemical Properties,Uses,Production
Description
Furazolidone belongs to the group of nitro furans. This antimicrobal (antibacterial and antiprotozoal) agent is used in veterinary medicine both topically and orally, particularly in animal feed. Reactions have been reported in workers exposed to it by contact with animal feed. Cross reactions with other nitrofuran derivatives are rare.
Chemical Properties
solid
Uses
The minimum inhibitory concentration of furazolidone was assessed to study the nonreplicating persistence of M. tuberculosis in aerobic and anaerobic conditions using luminescence-based low-oxygen-recovery assay.
Definition
ChEBI: A member of the class of oxazolidines that is 1,3-oxazolidin-2-one in which the hydrogen attached to the nitrogen is replaced by an N-{[(5-nitro-2-furyl)methylene]amino} group. It has antibacterial and antiprotozoal properties, and is us d in the treatment of giardiasis and cholera.
World Health Organization (WHO)
Furazolidone, a nitrofuran derivative with antibacterial and antiprotozoal activity, was introduced in 1954. In the 1970s it was shown to have a carcinogenic potential following long-term administration to experimental animals. However, the relevance of this to short-term therapy in man has not been established. The risk-benefit assessment varies and furazolidone remains widely available in many countries for the treatment of diarrhoea and enteritis.
Antimicrobial activity
It is active against a wide range of enteric pathogens, including Salmonella enterica, Shigella spp., enterotoxigenic Escherichia coli, Campylobacter jejuni, Aeromonas hydrophila, Plesiomonas shigelloides, Vibrio cholerae and V. parahaemolyticus. Yersinia enterocolitica is intrinsically resistant. Furazolidone is also active against the protozoa Giardia lamblia and Trichomonas vaginalis.
Acquired resistance
Acquired resistance has been observed in V. cholerae O1 and O139, S. enterica serotypes Typhi and Enteritidis, A. hydrophila and Shigella spp. Such resistance may be transferable, and there is cross-resistance with nitrofurantoin. Many of these reports come from the Indian subcontinent, where furazolidone is used widely for treating diarrheal diseases.
General Description
Furazolidone is an effective antiprotozoal and antibacterial agent.
Hazard
A questionable carcinogen, use has been restricted.
Pharmaceutical Applications
A non-ionic synthetic compound, available for oral use only. It is poorly soluble in water (40 mg/L) and ethanol (90 mg/L), but dissolves well in dimethylformamide (10 g/L). It decomposes in the presence of alkali.
Contact allergens
Furazolidone belongs to the group of nitrofurans. This antimicrobial (antibacterial and antiprotozoal) agent is used in veterinary medicine both topically and orally, particularly in animal feed. Reactions are reported in workers exposed to it in animal feeds. Cross-reactions with other nitrofuran derivatives are rare.
Pharmacokinetics
There is substantial absorption (65–70%) after oral administration, but the drug is heavily metabolized, so that only about 5% of the material excreted is microbiologically active. A dose of 5 mg/kg achieves a maximum plasma concentration of around 1 mg/L. Protein binding is about 30%. Intact drug can be found in various body fluids in concentrations approximating to the minimum inhibitory concentration (MIC) for various intestinal pathogens. Less than 1% of the drug is excreted into urine.
Clinical Use
Furazolidone is used in gastrointestinal infections and vaginitis. It is mainly used in developing countries to treat diarrheal diseases of varying etiology, but it is not the drug of choice if a specific pathogen has been identified. Use as a secondline agent in giardiasis and as part of multidrug regimens in Helicobacter infection has been advocated.
Side effects
Most reported side effects are mild and only rarely cause discontinuation of treatment. Nausea and vomiting are experienced by around 8% of patients. Other adverse events include neurological reactions (mainly headache; 1.3% of patients), ‘systemic’ reactions such as fever and malaise (0.6%) and skin rashes (0.54%). Administration of furazolidone may give rise to inhibition of monoamine oxidase, and disulfiram-like reactions have been reported.
Safety Profile
Poison by ingestion and intraperitoneal routes. Human systemic effects by ingestion: dyspnea, respiratory depression, and eosinophilta. Experimental reproductive effects. Human mutation data reported. Questionable carcinogen. When heated to decomposition it emits toxic fumes of NOx.
Furazolidone Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Medi Pharma Drug House | +919930911911 | Mumbai, India | 143 | 58 | Inquiry |
| CRYSTAL PHARMA | +91-9322218073 +91-9820141331 | Mumbai, India | 106 | 58 | Inquiry |
| Besil Chem LLP | +91-7760252666 +91-9880731835 | Bangalore, India | 163 | 58 | Inquiry |
| Lanya Chem Industries Pvt Ltd | +91-7290016802 +91-9996207397 | Haryana, India | 34 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Bhavi Chem | 08048602333 | Mumbai, India | 73 | 58 | Inquiry |
| Animed | 08069013892Ext 829 | Kolkata, India | 7 | 58 | Inquiry |
| Labdhi Chemicals | 08046034928 | Ahmedabad, India | 69 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Medi Pharma Drug House | 58 |
| CRYSTAL PHARMA | 58 |
| Besil Chem LLP | 58 |
| Lanya Chem Industries Pvt Ltd | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Pharmaffiliates Analytics and Synthetics P. Ltd | 58 |
| Central Drug House(P) Ltd. | 58 |
| Bhavi Chem | 58 |
| Animed | 58 |
| Labdhi Chemicals | 58 |
Related articles
- Uses of Furazolidone
- This nitrofuran has in vitro activity against S. aureus, S. pyogenes, Enterococcus faecalis, E. coli, Vibrio cholerae, Clostri....
- Mar 10,2022
67-45-8(Furazolidone)Related Search:
1of4
chevron_right




