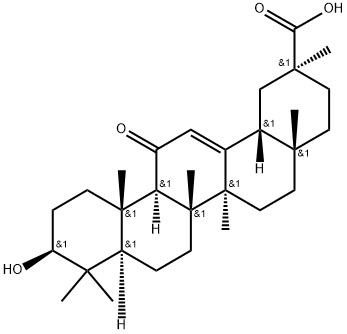
18β-Glycyrrhetinic Acid
- CAS No.
- 471-53-4
- Chemical Name:
- 18β-Glycyrrhetinic Acid
- Synonyms
- GLYCYRRHETINIC ACID;ENOXOLONE;GLYCYRRHETIC ACID;Glycyrrhetinic;Glycyrrhetinate;biosone;URALENIC ACID;18β-Glycyrrhetic acid;18B-GLYCYRRHETINIC ACID;(2S,4aS,6aR,6aS,6bR,8aR,10S,12aS,14bR)-10-hydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-3,4,5,6,6a,7,8,8a,10,11,12,14b-dodecahydro-1H-picene-2-carboxylic acid
- CBNumber:
- CB8474793
- Molecular Formula:
- C30H46O4
- Molecular Weight:
- 470.69
- MOL File:
- 471-53-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/24 17:31:20
| Melting point | 292-295 °C(lit.) |
|---|---|
| alpha | 165 º (c=1, CHCl3,on dry ba) |
| Boiling point | 492.11°C (rough estimate) |
| Density | 0.9967 (rough estimate) |
| vapor pressure | 10-220Pa at 20-50℃ |
| refractive index | 162 ° (C=1, MeOH) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in ethanol, sparingly soluble in methylene chloride. |
| pka | pKa 5.56±0.1 (Uncertain) |
| form | Crystalline Powder |
| color | White to off-white |
| optical activity | [α]22/D +170.0°, c = 1 in chloroform |
| Water Solubility | SOLUBLE IN ACETIC ACID |
| Merck | 14,3590 |
| BRN | 2229654 |
| LogP | 4.5 at 20℃ |
| CAS DataBase Reference | 471-53-4(CAS DataBase Reference) |
| EPA Substance Registry System | Enoxolone (471-53-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H302 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P270-P301+P312-P330-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RK0180000 | |||||||||
| HS Code | 29189900 | |||||||||
| Toxicity | mouse,LD50,intraperitoneal,308mg/kg (308mg/kg),Drugs in Japan Vol. -, Pg. 319, 1990. | |||||||||
| NFPA 704 |
|
18β-Glycyrrhetinic Acid price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | G10105 | 18β-Glycyrrhetinic acid 97% | 471-53-4 | 10G | ₹15458.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | G10105 | 18β-Glycyrrhetinic acid 97% | 471-53-4 | 25G | ₹19052 | 2022-06-14 | Buy |
| TCI Chemicals (India) | G0149 | Glycyrrhetic Acid | 471-53-4 | 1G | ₹2300 | 2022-05-26 | Buy |
| TCI Chemicals (India) | G0149 | Glycyrrhetic Acid | 471-53-4 | 25G | ₹10700 | 2022-05-26 | Buy |
18β-Glycyrrhetinic Acid Chemical Properties,Uses,Production
Description
Extracting from liquorice (Glycyrrhiza uralensis Fisch, Gan Cao), glycyrrhetic acid also can be detected in other plants, such as Abrus cantoniensis Hance and Herba Abri fruticulosi. As one of traditional Chinese medicines, liquorice has been applied clinically for a long period. Due to its extensive usage, it plays an extremely important role in traditional Chinese medicine formula mainly as “guide” drug. As is recorded in Shen Nong’s Herbal Classic and later in pharmaceutical monographs, liquorice is able to strengthen bones and muscles and enhance metabolism and detoxification. Also, abnormal symptoms of the body and wound can be improved. Glycyrrhetic acid, the most important and potent ingredient of liquorice, has been recorded in Pharmacopoeia of the People’s Republic of China.
Chemical Properties
white or greyish-white crystalline powder
Physical properties
Solubility: insoluble in water; it exists in crystal with methanol and chloroform. Melting point: the compound melts at 292–295?°C. Specific optical rotation: under the condition of 20?°C, 589.3?nm, and 1?dm, polarized light rotates at 68° when it passes through the chloroform with a concentration of 64? mol/L.? Both 18α-glycyrrhetic acid and 18β-glycyrrhetic acid are chiral isomers of glycyrrhetic acid.
History
Glycyrrhetic acid originates from hydrolysis of glycyrrhizin, which has a therapeutic effect on disease. Dating back to the 1930s, the chemical structure of glycyrrhetic acid was demonstrated . Subsequently, the discovery of antiulcer activity promotes following research . The ramification of glycyrrhetic acid, carbenoxolone sodium, has a therapeutic effect on ulcer. In 2010, followed by the approval of raw materials, batches of tablets and capsules were approved in 2009, respectively. In foreign countries, 18β-glycyrrhetic acid was studied for anti-inflammatory effect on arthritis, rheumatoid disease, and periodontitis in BioNetWorks. The company applied for the patent of 18β-glycyrrhetic acid in 1999. Also, after joining the leading worldwide market in 2006, phase III clinical trials would be carried out in 2007. However, the progress was hindered in 2008. To detecting more indications, its carbenoxolone sodium was studied by other three companies: after conducting phase III clinical trials in the UK, the project of RB intending to improve nonspecific inflammatory bowel disease was given up in 1992. York Pharma expected to make progress in psoriasis with gel or cream; however, the project has been in a standstill after phase II clinical trials was conducted from 2005 to 2009. Canada pharmaceutical company, Oxalys Pharmaceuticals, research it for treating Huntington’s disease, and it was included in the orphan drug list by the USA in 2014. Till now, phase I clinical trials are still continuing.
Uses
The product may be used as a starting material to prepare 18β-glycyrrhetinic acid derivatives, which show anti-inflammatory and antioxidant properties.
Indications
Treatment of Addison’s disease, deoxycorticosterone
Definition
ChEBI: A pentacyclic triterpenoid that is olean-12-ene substituted by a hydroxy group at position 3, an oxo group at position 11 and a carboxy group at position 30.
General Description
18β-Glycyrrhetinic acid is a pentacyclic triterpenoid found in the Glycyrrhiza glabra L.(liquorice) roots. It is the key metabolite of glycyrrhizin and glycyrrhizic acid.
Pharmacology
Thirty percent of glycyrrhetinic acid can be effectively used by the body; both 18α-glycyrrhetic acid and 18β-glycyrrhetic acid reduce by half in 2.24 h and 11.5 h separately. CYP3A promotes metabolism with hydroxyl added to 22α and 24α .There are lots of pharmacological activities : it plays an anti-inflammatory role by inhibiting the activity of phospholipase A2 and lipoxygenase to reduce mediators of inflammation; the compound promotes antiulcer activity through the production of more PGE2 and secretion of gastric mucus; it also provokes proliferation of gastric cell to protect the mucosa from ulceration. The complex which consists of glycyrrhetinic acid and carotenoid plays antioxidation by scavenging free radical. Glycyrrhetinic acid inhibits the replication of viral DNA to achieve an antiviral effect at the concentration of 4×10?5 mol/L; it also inhibits proliferation of tumor cell and promotes apoptosis and differentiation. The decreasing ability of invasion exerts an antitumor effect. Glycyrrhetinic acid is considered to have extensive antiarrhythmic effects through inhibition of L-type calcium channel. In addition, glycyrrhetinic acid functions as an anticholinesterase (1.7×10?5 mol/L), anticoagulant, and antitetanus toxin; it also improves inner ear hearing (100 mg/kg, intramuscular injection) and improves absorption of insulin.
Clinical Use
Glycyrrhetic acid has not been applied in clinical treatment till now. Meanwhile, the ramification has come into the market for the property of antiulcer. However, with large doses and long-term usage, the drug gives rise to hypertension, sodium retention, and hypokalemia. When renin-angiotensin-aldosterone system fails to function properly, liquorice-induced pseudoaldosteronism threatens human health .
Anticancer Research
Glycyrrhetinic acid in combinationwith etoposide inhibits thetopoisomerase 2α and inducesapoptosis
Cai et al.(2017)
18β-Glycyrrhetinic Acid Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Alchem International Pvt Ltd | +91-1126809882 +91-1126809882 | New Delhi, India | 15 | 58 | Inquiry |
| Natural Remedies Pvt Ltd | +91 (80) 4020-9999 | New Delhi, India | 110 | 50 | Inquiry |
| Rita Corporation | 08048372139Ext 895 | Mumbai, India | 50 | 58 | Inquiry |
| Tashima Inc. | +91-802654 4588 | Bangalore, India | 19 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Hindustan Herbals Limited | 09813618312 | Haryana, India | 9 | 58 | Inquiry |
| Mehta Pharmaceuticals Private Limited | 09888047778 | Punjab, India | 1 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Ralington Pharma | 58 |
| Alchem International Pvt Ltd | 58 |
| Natural Remedies Pvt Ltd | 50 |
| Rita Corporation | 58 |
| Tashima Inc. | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Hindustan Herbals Limited | 58 |
| Mehta Pharmaceuticals Private Limited | 58 |
| SynZeal Research Pvt Ltd | 58 |
| Clearsynth Labs | 58 |
471-53-4(18β-Glycyrrhetinic Acid)Related Search:
1of4
chevron_right




