Sorafenib
- CAS No.
- 284461-73-0
- Chemical Name:
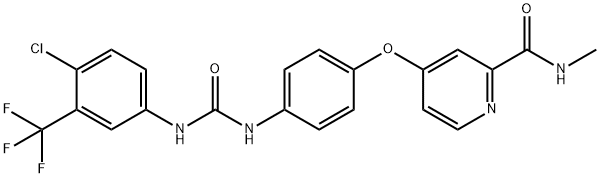
- Sorafenib
- Synonyms
- sorafenib;Nexavar;SORAFENIB-D3;BAY 43-9006;Sorafenib Free Base;4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-N-methylpicolinamide;N-[4-Chloro-3-(trifluoromethyl)phenyl]-({4-[2-(N-methyl-carbamoyl)(4-pyridyloxy)]phenyl}amino)-carboxamide;SL-API;Sorafini;Forafenib
- CBNumber:
- CB9252510
- Molecular Formula:
- C21H16ClF3N4O3
- Molecular Weight:
- 464.82
- MOL File:
- 284461-73-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/23 18:28:04
| Melting point | 202-204°C |
|---|---|
| Boiling point | 523.3±50.0 °C(Predicted) |
| Density | 1.454±0.06 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| pka | 12.89±0.70(Predicted) |
| form | White to off-white solid. |
| color | White to Off-White |
| Water Solubility | 100μg/L at 20℃ |
| LogP | 3.3 at 25℃ |
| CAS DataBase Reference | 284461-73-0(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
| Risk Statements | 68/20/21/22-37/38 |
| Safety Statements | 36-37-39 |
| HS Code | 29350090 |
Sorafenib Chemical Properties,Uses,Production
Description
Sorafenib is a small molecular inhibitor of several kinases involved in tumor angiogenesis and proliferation, including, but not limited to, Raf (IC50=12nM for Raf-1), VEGFR (IC50=90nM for VEGFR-2 and IC50=12nM for VEGFR-3), and platelet derived growth factor receptor (IC50=57nM for PDGFR-b). Specifically, sorafenib blocks tumor progression by inhibiting cellular proliferation that is dependent on activation of the MAPK pathway (Raf) and/or inhibiting tumor angiogenesis through VEGFR and/or PDGFR. While it may be effective in the treatment of a variety of tumors, the first approvable indication is for renal cell carcinoma. Overall, the drug appears to be well tolerated by the majority of patients at the 400 mg b.i.d. continuous dosing. As an inhibitor of multiple kinases vital for tumor progression, sorafenib may possess wide-spectrum antitumor properties and may emerge as an effective weapon against a variety of solid tumors.
Chemical Properties
Light Yellow Solid
Characteristics
Class: receptor tyrosine kinase
Treatment: RCC, HCC, thyroid cancer
Elimination half-life = 25–48 h
Protein binding = 99.7%
Characteristics
The complex of sorafenib with a nonphosphorylated VEGFR2 construct comprising the catalytic and juxtamembrane (JM) domain shows the activation loop adopts a DFG-out position. The net effect of sorafenib’s interactions with the kinase is to stabilize the DFG motif in an inactive conformation. The lipophilic trifluoromethyl phenyl ring at the opposite end of the molecule inserts into a hydrophobic pocket formed between the -C and -E helices and amino-terminal regions of the DFG motif and catalytic loop. The urea functionality forms two crucial hydrogen bonds, one with the backbone aspartate of the DFG loop and the other with the glutamate side chain of the -C helix. In the auto-inhibited state, the DFG-out segment, particularly Phe1047, blocks ATP-binding. When the JM rearranges out of the regulatory domain pocket (RDP), then the DFG is unlocked and able to switch to the open activated state. Autophosphorylation, which activates the kinase presumably through stabilization of an active conformation, was shown to occur on the JM and then the DFG-containing activation loop sequentially. Sorafenib fills the binding channel, making an important H-bond to Asp1046, two H-bonds to the side chain carboxylate oxygen of Glu885, and two H-bonds to Cys919. The selectivity of sorafenib derives from the formation of these four hydrogen bonds and attractive van der Waals (dispersion) interactions.
Uses
Sorafenib Tosylate (Bay 43-9006, Nexavar) is a small molecular inhibitor of VEGFR, PDGFR, c-Raf and B-Raf with IC50s of 18 nM, 10 nM, 3 nM and 15 nM, respectively.
Indications
Sorafenib (Nexavar(R), Bayer) was the first approved inhibitor targeting the vascular endothelial growth factor (VEGF) family kinases, which include VEGFR1, VEGR2, and VEGFR3. Sorafenib was originally approved for the treatment of renal cell carcinoma (RCC) in 2005, hepatocellular carcinoma in 2007, and locally recurrent or metastatic thyroid carcinoma refractory to radioactive iodine treatment in 2013. Six other approved inhibitors with VEGFRs as the main targets are sunitinib (Sutent(R), Pfizer) for RCC, soft tissue sarcoma, thyroid cancer,metastatic pancreatic tumors, gastrointestinal stromal tumor, and several other types of carcinomas; pazopanib (Votrient(R), GlaxoSmithKline) for RCC, soft tissue sarcoma, and thyroid cancer; axitinib (Inlyta(R), Pfizer) for RCC,thyroid cancer, and aplastic anemia, as well as T315I-mutant Bcr–Abl1-driven leukemia; regorafenib (Stivarga(R), Bayer) for gastrointestinal stromal tumors and colorectal cancer; nintedanib (Ofev(R), Boehringer Ingelheim) for the non-oncological indication of idiopathic pulmonary fibrosis; and lenvatinib (Lenvima(R), Eisai Inc.) for RCC and different types of thyroid cancers. Sunitinib, pazopanib, and lenvatinib bind to the “DFG-in”conformation of VEGFRs, while axitinib, regorafenib, and nintedanib bind to inactive VEGFRs adopting the “DFG-out”conformation.
Definition
ChEBI: Sorafenib is a member of the class of phenylureas that is urea in which one of the nitrogens is substituted by a 4-chloro-3-trifluorophenyl group while the other is substituted by a phenyl group which, in turn, is substituted at the para position by a [2-(methylcarbamoyl)pyridin-4-yl]oxy group. It has a role as an antineoplastic agent, an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor, a tyrosine kinase inhibitor, an angiogenesis inhibitor, an anticoronaviral agent and a ferroptosis inducer. It is a pyridinecarboxamide, a member of monochlorobenzenes, an aromatic ether, a member of (trifluoromethyl)benzenes and a member of phenylureas.
Sorafenib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Bulat Pharmaceutical Pvt Ltd | +91-8448085659 +91-8448085660 | Haryana, India | 123 | 58 | Inquiry |
| CDYMAX | +91-9019317788 +91-9019317788 | Bangalore, India | 28 | 58 | Inquiry |
| Sakar Healthcare | +91-8976292690 +91-9967572302 | Gujarat, India | 47 | 58 | Inquiry |
| Humble Healthcare Limited | +91-9720093000 +91-8006400378 | Uttar Pradesh, India | 141 | 58 | Inquiry |
| Vishrudh laboratories pvt ltd | +91-9666132889 +91-8097369002 | Telangana, India | 117 | 58 | Inquiry |
| Shreyaa Medilife Private Limited | +91-6354474696 +91-9879513108 | Ahmedabad, India | 67 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| Bulat Pharmaceutical Pvt Ltd | 58 |
| CDYMAX | 58 |
| Sakar Healthcare | 58 |
| Humble Healthcare Limited | 58 |
| Vishrudh laboratories pvt ltd | 58 |
| Shreyaa Medilife Private Limited | 58 |
| Aspen Biopharma Labs Pvt Ltd | 58 |
| A.J Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
Related articles
- The brief introduction of Sorafenib
- Sorafenib is a multikinase inhibitor that has recently obtained Food and Drug Administration (FDA) approval for the treatment ....
- Apr 1,2024
- Sorafenib: A kinase inhibitor
- Sorafenib is a medicine that targets proteins in cancer cells and stops the cancer cells from growing. It is used to treat liv....
- Feb 15,2023
284461-73-0(Sorafenib)Related Search:
1of4
chevron_right




